Summary
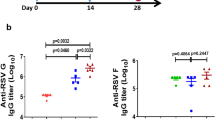
Respiratory syncytial virus (RSV) infection is the primary cause of respiratory disease in infants. The formalin-inactivated RSV (FI-RSV) vaccine resulted in an enhanced respiratory disease (ERD) in infants upon natural RSV infection, which is a major obstacle for development of safe and efficacious vaccines. Excessive and uncontrolled Th immune responses could be involved in the ERD. Agonists of TLRs are used as adjuvants to guide the type of immune response induced by vaccines. We evaluated the impact of lipopolysaccharide (LPS), the agonist of TLR4, on ERD as the adjuvant of FI-RSV. The results showed that LPS remarkably inhibited FI-RSV-enhanced lung inflammation, mucus production, airway inflammatory cell infiltration, and inflammatory cytokines following RSV challenge. Interestingly, LPS inhibited both Th2 and Th17 type cytokines in lungs of FI-RSV-immunized mice following RSV challenge, without an increase in the Th1 type cytokines, suggesting a controlled immune response. In contrast, Pam3Cys and Poly(I:C), the agonist of TLR1/2 or TLR3, partly inhibited FI-RSV-enhanced lung inflammation. Pam3Cys inhibited Th17 type cytokine IL-17, but promoted both Th1 and Th2 type cytokines. Poly(I:C) inhibited Th2 and Th17 type cytokines, but promoted Th1 type cytokines. In addition, LPS promoted IgG and IgG2a antibody production, which might provide protection from RSV challenge. These results suggest that LPS inhibits ERD without impairment in antibody production and protection, and the mechanism appears to be related with regulation of Th responses induced by FI-RSV.
Similar content being viewed by others
References
Glezen WP, Taber LH, Frank AL, et al. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child, 1986,140(6):543–546
Falsey AR, Hennessey PA, Formica MA, et al. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med, 2005,352(17):1749–1759
Kapikian AZ, Mtchell RH, Chanock RM, et al. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol, 1969,89(4):405–421
Prince GA, Curtis SJ, Yim KC, et al. Vaccine-enhanced respiratory syncytial virus disease in cotton rats following immunization with Lot 100 or a newly prepared reference vaccine. J Gen Virol, 2001,82(12):2881–2888
Graham BS, Henderson GS, Tang YW, et al. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. J Immunol, 1993,151(4):2032–2040
Zeng R, Zhang H, Hai Y, et al. Interleukin-27 inhibits vaccine-enhanced pulmonary disease following respiratory syncytial virus infection by regulating cellular memory responses. J Virol, 2012,86(8):4505–4517
Zhang L, Li H, Hai Y, et al. CpG in combination with an inhibitor of Notch signaling suppresses FI-RSV-enhanced airway hyperresponsiveness and inflammation through inhibiting Thl7 memory responses and promoting tissue resident memory cells in lungs. J Virol, 2017,91(10):e02111–e02116
Brentano F, Kyburz D, Schorr O, et al. The role of Tolllike receptor signalling in the pathogenesis of arthritis. Cell Immunol, 2005,233(2):90–96
Murphy BR, Olmsted RA, Collins PL, et al. Passive transfer of respiratory syncytial virus (RSV) antiserum suppresses the immune response to the RSV fusion (F) and large (G) glycoproteins expressed by recombinant vaccinia viruses. J Virol, 1988,62(10):3907
Prince GA, Hemming VG, Horswood RL, et al. Immunoprophylaxis and immunotherapy of respiratory syncytial virus infection in the cotton rat. Virus Res, 1985,3(3):193–206
Mataharo V, Cekic C, Martin M, et al. The Vaccine Adjuvant Monophosphoryl Lipid A as a TRIF-Biased Agonist of TLR4. Science, 2007,316(5831):1628–1632
Rose P. Innate immune recognition of viral infection. Uirusu, 2006,56(1): 1–8
Trudel M, Nadon F, Seguin C, et al. Protection of BALB/c mice from respiratory syncytial virus infection by immunization with a synthetic peptide derived from the G glycoprotein. Virology, 1991,185(2):749–757
Gershwin LJ, Dungworth DL, Himes SR, et al. Immunoglobulin E responses and lung pathology resulting from aerosol exposure of calves to respiratory syncytial virus and Mcropolyspora faeni. Int Arch Allergy Imm, 1990,92(3):293–300
Kalina WV, Woolums AR, Berghaus RD, et al. Formalin-inactivated bovine RSV vaccine enhances a Th2 mediated immune response in infected cattle. Vaccine, 2004,22(11):1465–1474
Mok H, Lee S, Utley TJ, et al. Venezuelan equine encephalitis virus replicon particles encoding respiratory syncytial virus surface glycoproteins induce protective mucosal responses in mice and cotton rats. J Virol, 2007,81(24):13710–13722
Mcnamara P, Ritson P, Selby A, et al. Bronchoalveolar lavage cellularity in infants with severe respiratory syncytial virus bronchiolitis. Arch Dis Child, 2003,88(10):922
Zeng R, Zhang ZX, Gong W, et al. Protective effect of a RSV subunit vaccine candidate G1F/M2 was enhanced by a HSP70-Like protein in mice. Biochem Bioph Res Co, 2008,377(2):495–499
Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy, 2005,4(3):281–286
Dobrovolskaia MA, Vogel SN. Toll receptors, CD14, and macrophage activation and deactivation by LPS. Mcrobes Infec, 2002,4(9):903–914
Fan H, Cook JA. Molecular mechanisms of endotoxin tolerance. J Endotoxin Res, 2004,10(2):71–84
Rieser C, Papesh C, Herold M, et al. Differential deactivation of human dendritic cells by endotoxin desensitization: role of tumor necrosis factor-alpha and prostaglandin E2. Blood, 1998,91(9):3112–3117
Koike K, Moore FA, Moore EE, et al. Endotoxin pretreatment inhibits neutrophil proliferation and function. J Surg Res, 1994,57(1):49–54
Cyr SL, Angers I, Guillot L, et al. TLR4 and MyD88 control protection and pulmonary granulocytic recruitment in a murine intranasal RSV immunization and challenge model. Vaccine, 2009,27(3):421–430
Patel M, Xu D, Kewin P, et al. TLR2 agonist ameliorates established allergic airway inflammation by promoting Thl response and not via regulatory T cells. J Immunol, 2005,174(12):7558–7563
Thomauszynski S, Kiertscher SM, Ochoa MT, et al. Activation of toll-like receptor 2 on human dendritic cells triggers induction of IL-12, but not IL-10. J Immunol, 2000,165(7):3804–3810
Redecke V, Hacker H, Datta SK, et al. Cutting Edge: Activation of Toll-Like Receptor 2 Induces a Th2 Immune Response and Promotes Experimental Asthma. J Immunol, 2004,172(5):2739
Longhi MP, Trumpfheller C, Idoyaga J, et al. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J Exp Med, 2009,206(7):1589–1602
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by grants from the National Natural Science Foundation of China (No. 81671635 and No. 31240084) and Natural Science Foundation of Hebei Province (No. H2016206473).
Rights and permissions
About this article
Cite this article
Yin, W., Li, Hy., Zheng, By. et al. Lipopolysaccharide Inhibits FI-RSV Vaccine-enhanced Inflammation Through Regulating Th Responses. CURR MED SCI 39, 363–370 (2019). https://doi.org/10.1007/s11596-019-2044-0
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-019-2044-0