Abstract
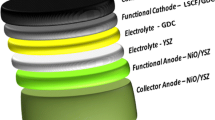
Solid oxide fuel cells based on yttria-stabilized zirconia materials have demonstrated a higher level of technology maturity compared to newer materials actively researched to lower the operating temperature. Yttria-stabilized zirconia-based cells can operate around 600 °C and achieve competitive power densities provided the electrolyte can be fabricated relatively thin. In this work, a 2.5-μm thick yttria-stabilized zirconia electrolyte, commercially available, anode-supported solid oxide fuel cell is systematically investigated under various electrochemical conditions, and area of improvements with the electrochemical performance are identified. The cell consists of a Ni-YSZ bulk and functional layer anode, YSZ electrolyte, GDC barrier layer, and LSCF cathode. Using humidified hydrogen, the peak power densities are determined to be 0.31, 0.58, 0.96, 1.41, and 1.78 W/cm2 at 600, 650, 700, 750, and 800 °C, respectively. It is found that the ceria barrier layer is porous; thus, it is not effective to avoid the formation of strontium zirconate. It is therefore expected that the performance can be improved further if a denser ceria barrier layer can be deposited. In addition, energy dispersive spectroscopy analysis revealed significant Ce/Zr interdiffusion between the barrier layer and the electrolyte.















Similar content being viewed by others
References
Minh N (2004) Solid oxide fuel cell technology—features and applications. Solid State Ionics 174:271–277
Singhal S (2002) Solid oxide fuel cells for stationary, mobile and military applications. Solid State Ionics 152:405–410
Lawlor V, Reissig M, Makinson J, Rechberger J (2017) SOFC system for battery electric vehicle range extension: results of the first half of the Mestrex project. ECS Trans 78(1):191–195
Kendall K, Liang B, Kendall M (2017) Microtubular SOFC (mSOFC) system in mobile robot applications. ECS Trans 78(1):237–242
Gao Z, Mogni L, Miller E, Railsback J, Barnett S (2016) A perspective on low-temperature solid oxide fuel cells. Energy Environ Sci 9:1602–1644
Wachsman E, Ball G, Jiang N, Stevenson D (1992) Structural and defect studies in solid oxide electrolytes. Solid State Ionics 52(1–3):213–218
Choi S, Yoo S, Kim J, Park S, Jun A, Sengodan S, Kim J, Shin J, Jeong HY, Choi Y, Kim G, Liu M (2013) Highly efficient and robust cathode materials for low-temperature solid oxide fuel cells: PrBa0.5Sr0.5Co2-xFexO5+δ. Sci Rep 3:2426–2432
Tucker M (2010) Progress in metal-supported solid oxide fuel cells: a review. J Power Sources 195:4570–4582
Chatzimichail R, Dawson R, Green S, Sullivan D, Mukerjee S, Selby M (2017) Engineering FEA sintering model development for metal supported SOFC. ECS Trans 78(1):2773–2783
Strandbakke R, Cherepanov V, Zuev A, Tsvetkov D, Argirusis C, Sourkouni G, Prünte S, Norby T (2015) Gd- and Pr-based double perovskite cobaltites as oxygen electrodes for proton ceramic fuel cells and electrolyser cells. Solid State Ionics 278:120–132
Duan C, Kee R, Zhu H, Karakaya C, Chen Y, Ricote S, Jarry A, Crumlin E, Hook D, Braun R, Sullivan N, O’Hayre R (2018) Highly durable, coking and sulfur tolerant, fuel-flexible protonic ceramic fuel cells. Nature 557:217–222
Minh N, Mizusaki J, Singhal S (2017) Advances in solid oxide fuel cells: review of progress through three decades of the international symposia on solid oxide fuel cells. ECS Trans 78(1):63–73
Fabbri E, Bi L, Pergolesi D, Traversa E (2012) Towards the next generation of solid oxide fuel cells operating below 600°C with chemically stable proton-conducting electrolytes. Adv Mater 24:195–208
Albrecht KJ, Braun RJ (2016) The effect of coupled mass transport and internal reforming on modeling of solid oxide fuel cells part I: channel-level model development and steady-state comparison. J Power Sources 304:384–401
Boukamp B (1995) A linear Kronig-Kramers transform test for immittance data validation. J Electrochem Soc 142:1885–1894
Schönleber M, Klotz D, Ivers-Tiffée E (2014) A method for improving the robustness of linear Kramers-Kronig validity tests. Electrochim Acta 131:20–27
Schönleber M, Ivers-Tiffée E (2015) Approximability of impedance spectra by RC elements and implications for impedance analysis. Electrochem Commun 58:15–19
Chen Y, Choi Y, Yoo S, Ding Y, Yan R, Pei K, Qu C, Zhang L, Chang I, Zhao B, Zhang Y, Chen H, Chen Y, Yang C, deGlee B, Murphy R, Liu J, Liu M (2018) A highly efficient multi-phase catalyst dramatically enhances the rate of oxygen reduction. Joule 2:1–12
Boukamp B (2015) Fourier transform distribution function of relaxation times; application and limitations. Electrochim Acta 154:35–46
Kromp A, Leonide A, Weber A, Ivers-Tiffée E (2011) Electrochemical analysis of reformate-fuelled anode supported SOFC. J Electrochem Soc 158(8):B980–B986
Wan T, Saccoccio M, Chen C, Ciucci F (2015) Influence of the discretization methods on the distribution of relaxation times deconvolution: implementing radial basis functions with DRTtools. Electrochim Acta 184:483–499
Johnson D (2016) ZView Electrochemical Impedence Software, version 3.5e. Scriber Associates, Inc., Southern Pines, NC
Zhu H, Kee R, Janardhanan V, Deutschmann O, Goodwin D (2005) Modeling elementary heterogeneous chemistry and electrochemistry in solid-oxide fuel cells. J Electrochem Soc 152:A2427–A2440
Gazzarri JI, Kesler O (2007) Non-destructive delamination detection in solid oxide fuel cells. J Power Sources 167:430–441
Swierczek K, Gozu M (2007) Structural and electrical properties of selected La1-xSrxCo0.2Fe0.8O3 and La0.6Sr0.4Co0.2Fe0.6Ni0.2O3 perovskite type oxide. J Power Sources 173:695–699
Gao Z, Zenou V, Kennouche D, Marks L, Barnett S (2015) Solid oxide cells with zirconia/ceria Bi-Layer electrolytes fabricated by reduced temperature firing. J Mater Chem A 3(18):9955–9964
Nguyen H, Hardy J, Coyle C, Lub Z, Stevenson J (2017) Developing cost-effective dense continuous SDC barrier layers for SOFCs. ECS Trans 75(42):107–114
Leonide A, Sonn V, Weber A, Ivers-Tiffée E (2008) Evaluation and modeling of the cell resistance in anode-supported solid oxide fuel cells. J Electrochem Soc 155(1):B36–B41
Muhammed Ali SA, Anwar M, Mahmud LS, Kalib NS, Muchtar A, Somalu MR (2019) Influence of current collecting and functional layer thickness on the performance stability of La0.6Sr0.4Co0.2Fe0.8O3-δ-Ce0.8Sm0.2O1.9 composite cathode. J Solid State Electrochem. https://doi.org/10.1007/s10008-019-04208-6
Minh N (2018) Innovative, versatile and cost-effective solid oxide fuel cell stack concept, 19thAnnual solid oxide fuel cell project review meeting, Washington, DC, June 13–15
Acknowledgements
This work was conducted at Nissan’s facilities in Farmington Hills, MI during a sabbatical leave from Kettering University by the corresponding author.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
DiGiuseppe, G., Thompson, D., Gumeci, C. et al. Impedance analysis of thin YSZ electrolyte for low-temperature solid oxide fuel cells. Ionics 25, 3537–3548 (2019). https://doi.org/10.1007/s11581-019-02935-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-019-02935-4