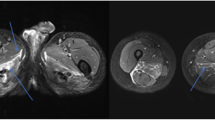
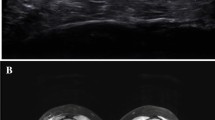
Abstract
Objective
To compare the MRI features in patients with fibrosing [FM] versus non-fibrosing [NFM] systemic sclerosis [SSc]-associated myopathy.
Methods
10 patients with FM and 14 with NFM underwent bilateral thigh MRI [T1-weighted, STIR and DW/ADC mapping]. Three observers, blinded to histology evaluated 36 muscles per patient for presence of intramuscular edema, fascial edema, fatty replacement and atrophy and measured ADC values. Fisher's exact test and student’s t-test were used to compare MRI findings of FM [endomysial/ perimysial fibrosis] and NFM [necrosis/inflammation] on histology.
Results
Intramuscular edema [p < 0.0001] and fascial edema [p = 0.07] were more common in FM. On DWI, elevated intramuscular signal was more common in FM, [low b-value: p < 0.0001 and high b-value: p < 0.0001]. On T1, NFM exhibited more fatty replacement [p = < 0.0001] and atrophy [p = < 0.0001].
Conclusions
Intramuscular and fascial edema on MRI are more common in SSc-associated FM, while markers of chronic muscle damage are more often associated with NFM.




Similar content being viewed by others
Code availability
N/A
References
van den Hoogen F, Khanna D, Fransen J et al (2013) classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum 65:2737–2747
Thompson JM, Bluestone R, Bywaters EG et al (1969) Skeletal muscle involvement in systemic sclerosis. Ann Rheum Dis 28:281–288
Muangchan C, Markland J, Robinson D et al (2013) The 15% rule in scleroderma: the frequency of severe organ complications in systemic sclerosis. A systematic review J Rheumatol 40:1545–1556
Averbuch-Heller L, Steiner I, Abramsky O (1992) Neurologic manifestations of progressive systemic sclerosis. Arch Neurol 49:1292–1295
West SG, Killian PJ, Lawless OJ (1981) Association of myositis and myocarditis in progressive systemic sclerosis. Arthritis Rheum 24:662–667
Russell ML, Hanna WM (1983) Ultrastructure of muscle microvasculature in progressive systemic sclerosis: relation to clinical weakness. J Rheumatol 10:741–747
Ranque B, Authier FJ, Berezne A et al (2007) Systemic sclerosis–associated myopathy. Ann N Y Acad Sci 1108:268–282
Paik JJ, Mammen AL, Wigley FM, Gelber AC (2014) Myopathy in scleroderma, its identification, prevalence, and treatment: lessons learned from cohort studies. Curr Opin Rheumatol 26(2):124–130
Jung M, Bonner A, Hudson M, Baron M, Pope J, on behalf of the Canadian Scleroderma Research Group (2014) Myopathy is a poor prognostic feature in systemic sclerosis: results from the Canadian Scleroderma Research Group [CSRG] cohort. Scand J Rheumatol 43:217–220
Follansbee WP, Zerbe TR, Medsger TA (1993) Cardiac and skeletal muscle disease in systemic sclerosis [scleroderma]: a high risk association. Am Heart J 125:194–203
Mimura Y, Ihn H, Jinnin M et al (2005) Clinical and laboratory features of scleroderma patients developing skeletal myopathy. Clin Rheumatol 24:99–102
Paik JJ, Wigley FM, Mejia AF, Hummers LK (2016) Independent Association of Severity of Muscle Weakness With Disability as Measured by the Health Assessment Questionnaire Disability Index in Scleroderma. Arthritis Care Res [Hoboken] 68:1695–1703
Paik JJ, Wigley FM, Lloyd TE, Corse AM, Casciola4-Rosen L, Shah AA, Boin F, Hummers LK, Mammen AL (2015) Spectrum of Muscle Histopathologic Findings in Forty-Two Scleroderma Patients With Weakness. Arthritis Care Res [Hoboken]. 67:1416-25.
Paik JJ, Wigley FM, Shah AA, Corse AM, Casciola-Rosen L, Hummers LK, Mammen AL (2017) Fibrosing myopathy in systemic sclerosis associates with higher mortality. Arthritis Care Res [Hoboken] 69(11):1764–1770. https://doi.org/10.1002/acr.23291
Dalakas MC (2015) Inflammatory muscle diseases. N Engl J Med 373:393–394
Kuo GP, Carrino JA (2007) Skeletal muscle imaging and inflammatory myopathies. Curr Opin Rheumatol 19:530–535
Van De Vlekkert J, Maas M, Hoogendijk JE et al (2015) Combining MRI and muscle biopsy improves diagnostic accuracy in subacute-onset idiopathic inflammatory myopathy. Muscle Nerve 51:253–258
Pinal-Fernandez I, Casal-Dominguez M, Carrino JA et al (2017) Thigh muscle MRI in immune-mediated necrotising myopathy: extensive oedema, early muscle damage and role of anti-SRP autoantibodies as a marker of severity. Ann Rheum Dis 76:681–687
Friedman SD, Poliachik SL, Otto RK et al (2014) Longitudinal features of STIR bright signal in FSHD. Muscle Nerve 49:257–260
Schanz S, Henes J, Ulmer A et al (2013) Magnetic resonance imaging findings in patients with systemic scleroderma and musculoskeletal symptoms. Eur Radiol 23:212–221
Tomasová Studynková J, Charvát F, Jarosová K, Vencovsky J (2007) The role of MRI in the assessment of polymyositis and dermatomyositis. Rheumatology [Oxford]. 46:1174–1179
Guimaraes JB, Zanoteli E, Link TM et al (2017) Sporadic Inclusion Body Myositis: MRI Findings and Correlation With Clinical and Functional Parameters. AJR Am J Roentgenol 209:1340–1347
Taouli B et al (2016) Diffusion-weighted imaging outside the brain: Consensus statement from an ISMRM-sponsored workshop. J Magn Reson Imaging 44:521–540
Meyer HJ, Ziemann O, Kornhuber M et al (2017) Apparent diffusion coefficient [ADC] does not correlate with different serological parameters in myositis and myopathy. Acta Radiol 59(6):694–699
Ran J, Liu Y, Sun D et al (2016) The diagnostic value of biexponential apparent diffusion coefficients in myopathy. J Neurol 263(1296–1302):7
Qi J, Olsen NJ, Price RR et al (2008) Diffusion-weighted imaging of inflammatory myopathies: polymyositis and dermatomyositis. J Magn Reson Imaging 27:212–217
Partovi S, Schulte AC, Aschwanden M et al (2012) Impaired skeletal muscle microcirculation in systemic sclerosis. Arthritis Res Ther 14:R209
Heemskerk AM, Drost MR, van Bochove GS et al (2006) DTI-based assessment of ischemia-reperfusion in mouse skeletal muscle. Magn Reson Med 56(2):272–281 (PubMed PMID: 16826605)
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Shivani Ahlawat has received support from Department of Defense 2018–2023 and Pfizer (2017–2018). Laura M. Fayad has received support from GERRAF 2008–10 and Siemens 2011–12. Julie Paik has received supported in part by Doris Duke Early Clinician Investigator Award, Rheumatology Research Foundation, K Bridge Award, and Stauralaukis Discovery Award. The remaining authors have no conflicts of interest.
Availability of data and material
Can be made available on request.
Ethics approval
This retrospective observational study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Human Investigation Committee (IRB) of Johns Hopkins University approved this study.
Consent to participate
The requirement for informed consent was waived.
Consent for publication
N/A
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahlawat, S., Paik, J., Del Grande, F. et al. Distinct MR features in scleroderma associated myopathy. Radiol med 126, 707–716 (2021). https://doi.org/10.1007/s11547-020-01317-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-020-01317-5