Abstract
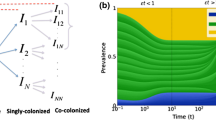
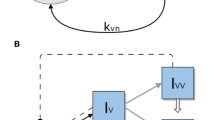
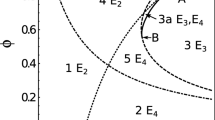
Humans are often colonized by polymorphic bacteria such as Streptococcus pneumoniae, Bordetella pertussis, Staphylococcus Aureus, and Haemophilus influenzae. Two co-colonizing pathogen clones may interact with each other upon host entry and during within-host dynamics, ranging from competition to facilitation. Here we examine the significance of these exploitation strategies for bacterial spread and persistence in host populations. We model SIS epidemiological dynamics to capture the global behavior of such multi-strain systems, focusing on different parameters of single and dual colonization. We analyze the impact of heterogeneity in clearance and transmission rates of single and dual colonization and find the criteria under which these asymmetries enhance endemic persistence. We obtain a backward bifurcation near \(R_0 = 1\) if the reproductive value of the parasite in dually infected hosts is sufficiently higher than that in singly infected ones. In such cases, the parasite is able to persist even in sub-threshold conditions, and reducing the basic reproduction number below 1 would be insufficient for elimination. The fitness superiority in co-colonized hosts can be attained by lowering net parasite clearance rate (\(\gamma _\mathrm{{d}}\)), by increasing transmission rate (\(\beta _\mathrm{{d}}\)), or both, and coupling between these traits critically constrains opportunities of pathogen survival in the \(R_0<1\) regime. Finally, using an adaptive dynamics approach, we verify that despite their importance for sub-threshold endemicity, traits expressed exclusively in coinfection should generally evolve independently of single infection traits. In particular, for \(\beta _\mathrm{{d}}\) a saturating parabolic or hyperbolic function of \(\gamma _\mathrm{{d}}\), co-colonization traits evolve to an intermediate optimum (evolutionarily stable strategy, ESS), determined only by host lifespan and the trade-off parameters linking \(\beta _\mathrm{{d}}\) and \(\gamma _\mathrm{{d}}\). Our study invites more empirical attention to the dynamics and evolution of parasite life-history traits expressed exclusively in coinfection.









Similar content being viewed by others
References
Adler FR, Brunet RC (1991) The dynamics of simultaneous infections with altered susceptibilities. Theor Popul Biol 40(3):369–410
Alizon S (2008) Decreased overall virulence in coinfected hosts leads to the persistence of virulent parasites. Am Nat 172(2):E67–E79
Alizon S (2013a) Co-infection and super-infection models in evolutionary epidemiology. Interface Focus 3:20130031
Alizon S (2013b) Parasite co-transmission and the evolutionary epidemiology of virulence. Evolution 67(4):921–933
Auranen K, Mehtala J, Tanskanen A, Kaltoft MS (2010) Between-strain competition in acquisition and clearance of pneumococcal carriage: epidemiologic evidence from a longitudinal study of day-care children. Am J Epidemiol 171(2):169–176
Balmer O, Tanner M (2011) Prevalence and implications of multiple-strain infections. Lancet Infect Dis 11(11):868–878
Bluestone CD (1996) Pathogenesis of otitis media: role of Eustachian tube. Pediatri Infect Dis J 15(4):281–291
Brauer F (2004) Backward bifurcations in simple vaccination models. J Math Anal Appl 298(2):418–431
Choisy M, de Roode JC (2010) Mixed infections and the evolution of virulence: effects of resource competition, parasite plasticity, and impaired host immunity. Am Nat 175(5):E105–E118
Cox F (2001) Concomitant infections, parasites and immune responses. Parasitology 122(S1):S23–S38
Dawid S, Roche AM, Weiser JN (2007) The blp bacteriocins of Streptococcus pneumoniae mediate intraspecies competition both in vitro and in vivo. Infect Immun 75(1):443–451
De Lencastre H, Kristinsson KG, Brito-Avô A, Sanches IS, Sá-Leão R, Saldanha J, Sigvaldadottir E, Karlsson S, Oliveira D (1999) Carriage of respiratory tract pathogens and molecular epidemiology of Streptococcus pneumoniae colonization in healthy children attending day care centers in Lisbon, Portugal. Microb Drug Resist 5(1):19–29
Diekmann O, Heesterbeek JAP (2000) Mathematical epidemiology of infectious diseases: model building, analysis and interpretation. Wiley, London
Dushoff J, Huang W, Castillo-Chavez C (1998) Backwards bifurcations and catastrophe in simple models of fatal diseases. J Math Biol 36(3):227–248
Eshel I (1983) Evolutionary and continuous stability. J Theor Biol 103(1):99–111
Feng Z, Castillo-Chavez C, Capurro AF (2000) A model for tuberculosis with exogenous reinfection. Theor Popul Biol 57(3):235–247
Gandon S (2004) Evolution of multihost parasites. Evolution 58(3):455–469
Geritz SA, Mesze G, Metz JA et al (1998) Evolutionarily singular strategies and the adaptive growth and branching of the evolutionary tree. Evol Ecol 12(1):35–57
Gjini E, Valente C, Sá-Leão R, Gomes MGM (2016) How direct competition shapes coexistence and vaccine effects in multi-strain pathogen systems. J Theor Biol 388:50–60
Greenhalgh D, Diekmann O, de Jong MC (2000) Subcritical endemic steady states in mathematical models for animal infections with incomplete immunity. Math Biosci 165(1):1–25
Huang W, Cooke KL, Castillo-Chavez C (1992) Stability and bifurcation for a multiple-group model for the dynamics of hiv/aids transmission. SIAM J Appl Math 52(3):835–854
Kadioglu A, Weiser JN, Paton JC, Andrew PW (2008) The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat Rev Microbiol 6(4):288–301
Leibovitch EC, Brunetto GS, Caruso B, Fenton K, Ohayon J, Reich DS, Jacobson S (2014) Coinfection of human herpesviruses 6A (HHV-6A) and HHV-6B as demonstrated by novel digital droplet PCR assay. PloS one 9(3):e92328
Lijek RS, Weiser JN (2012) Co-infection subverts mucosal immunity in the upper respiratory tract. Curr Opin Immunol 24(4):417–423
Lion S (2013) Multiple infections, kin selection and the evolutionary epidemiology of parasite traits. J Evol Biol 26(10):2107–2122
Lipsitch M, Abdullahi O, DÁmour A, Xie W, Weinberger DM, Tchetgen ET, Scott JAG (2012) Estimating rates of carriage acquisition and clearance and competitive ability for pneumococcal serotypes in Kenya with a Markov transition model. Epidemiology (Cambridge, Mass.) 23(4):510
Lipsitch M, Dykesa J, Johnsona S, Adesa E, Kingc J, Brilesc D, Carlonea G et al (2000) Competition among Streptococcus pneumoniae for intranasal colonization in a mouse model. Vaccine 19(4):598
Lord C, Barnard B, Day K, Hargrove J, McNamara J, Paul R, Trenholme K, Woolhouse M (1999) Aggregation and distribution of strains in microparasites. Philos Trans R Soc B Biol Sci 354(1384):799–807
Lysenko ES, Ratner AJ, Nelson AL, Weiser JN (2005) The role of innate immune responses in the outcome of interspecies competition for colonization of mucosal surfaces. PLoS Pathog 1(1):e1
Margolis E, Yates A, Levin BR (2010) The ecology of nasal colonization of Streptococcus pneumoniae, Haemophilus influenzae and Staphylococcus aureus: the role of competition and interactions with host’s immune response. BMC Microbiol 10(1):59
Marks LR, Reddinger RM, Hakansson AP (2012) High levels of genetic recombination during nasopharyngeal carriage and biofilm formation in Streptococcus pneumoniae. mBio 3(5):e00200-12. doi:10.1128/mBio.00200-12
Masuda K, Masuda R, Nishi J-I, Tokuda K, Yoshinaga M, Miyata K (2002) Incidences of nasopharyngeal colonization of respiratory bacterial pathogens in Japanese children attending day-care centers. Pediatr Int 44(4):376–380
McNally L, Viana M, Brown SP (2014) Cooperative secretions facilitate host range expansion in bacteria. Nat Commun 5:4594. doi:10.1038/ncomms5594
Méthot P-O, Alizon S (2014) What is a pathogen? toward a process view of host-parasite interactions. Virulence 5(8):775–785
Metz JA, Nisbet RM, Geritz SA (1992) How should we define ‘fitness’ for general ecological scenarios? Trends Ecol Evol 7(6):198–202
Mook-Kanamori BB, Geldhoff M, van der Poll T, van de Beek D (2011) Pathogenesis and pathophysiology of pneumococcal meningitis. Clin Microbiol Rev 24(3):557–591
Murphy TF, Bakaletz LO, Smeesters PR (2009) Microbial interactions in the respiratory tract. Pediatr Infect Dis J 28(10):S121–S126
Read AF, Taylor LH (2001) The ecology of genetically diverse infections. Science 292(5519):1099–1102
Riley MA, Gordon DM (1999) The ecological role of bacteriocins in bacterial competition. Trends Microbiol 7(3):129–133
Shak JR, Vidal JE, Klugman KP (2013) Influence of bacterial interactions on pneumococcal colonization of the nasopharynx. Trends Microbiol 21(3):129–135
Sharp GB, Kawaoka Y, Jones DJ, Bean WJ, Pryor SP, Hinshaw V, Webster RG (1997) Coinfection of wild ducks by influenza a viruses: distribution patterns and biological significance. J Virol 71(8):6128–6135
Smith J, Aberle JH, Fleming VM, Ferenci P, Thomson EC, Karayiannis P, McLean AR, Holzmann H, Klenerman P (2010) Dynamic coinfection with multiple viral subtypes in acute hepatitis C. J Infect Dis 202(12):1770–1779
van Baalen M, Sabelis MW (1995) The dynamics of multiple infection and the evolution of virulence. Am Nat 146:881–910
Van den Driessche P, Watmough J (2002) Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math Biosci 180(1):29–48
Wang W (2006) Backward bifurcation of an epidemic model with treatment. Math Biosci 201(1):58–71
Weinberger DM, Malley R, Lipsitch M (2011) Serotype replacement in disease after pneumococcal vaccination. Lancet 378(9807):1962–1973
Wolfram Research, Inc., (2011) Mathematica 8.0. https://www.wolfram.com
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gaivão, M., Dionisio, F. & Gjini, E. Transmission Fitness in Co-colonization and the Persistence of Bacterial Pathogens. Bull Math Biol 79, 2068–2087 (2017). https://doi.org/10.1007/s11538-017-0320-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-017-0320-3