Abstract
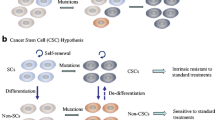
We use a mathematical model to investigate cancer resistance to radiation, based on dedifferentiation of non-stem cancer cells into cancer stem cells. Experimental studies by Iwasa 2008, using human non-small cell lung cancer (NSCLC) cell lines in mice, have implicated the inhibitor of apoptosis protein survivin in cancer resistance to radiation. A marked increase in radio-sensitivity was observed, after inhibiting survivin expression with a specific survivin inhibitor YM155 (sepantronium bromide). It was suggested that these observations are due to survivin-dependent dedifferentiation of non-stem cancer cells into cancer stem cells. Here, we confirm this hypothesis with a mathematical model, which we fit to Iwasa’s data on NSCLC in mice. We investigate the timing of combination therapies of YM155 administration and radiation. We find an interesting dichotomy. Sometimes it is best to hit a cancer with a large radiation dose right at the beginning of the YM155 treatment, while in other cases, it appears advantageous to wait a few days until most cancer cells are sensitized and then radiate. The optimal strategy depends on the nature of the cancer and the dose of radiation administered.











Similar content being viewed by others
References
Abratt RP, Bogart JA, Hunter A (2002) Hypofractionated irradiation for non-small cell lung cancer. Lung Cancer 36(3):225–233
Ailles LE, Weissman IL (2007) Cancer stem cells in solid tumors. Curr Opin Biotechnol 18(5):460–466
Al-Hajj M, Wicha MS, Benito-Hernandez A et al (2003) Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 100(7):3983–3988
Alpen E (1997) Radiation biophysics, 2nd edn. Academic Press, Cambridge
Altieri DC (2003) Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene 22:8581–8589
Amini A, Lin SH, Wei C et al (2012) Accelerated hypofractionated radiation therapy compared to conventionally fractionated radiation therapy for the treatment of inoperable non-small cell lung cancer. Radiat Oncol. doi:10.1186/1748-717X-7-33
Bachman JWN, Hillen T (2013) Mathematical optimization of the combination of radiation and differentiation therapies for cancer. Front Oncol. doi:10.3389/fonc.2013.00052
Badri H, Pitter K, Holland EC et al (2015) Optimization of radiation dosing schedules for proneural glioblastoma. J Math Biol. doi:10.1007/s00285-015-0908-x
Bao S, Wu Q, McLendron RE et al (2006) Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444(7120):756–760
Borsi I, Fasano A, Primicerio M et al (2015) A non-local model for cancer stem cells and the tumor growth paradox. Math Med Biol. doi:10.1093/imammb/dqv037
Burnham KP, Anderson DR, Huyvaert KP (2011) AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behav Ecol Sociobiol 65(1):23–35
Chaffer CL, Brueckmann I, Scheel C et al (2011) Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc Natl Acad Sci 108(19):7950–7955
Dahan P, Martinez Gala J, Delmas C et al (2014) Ionizing radiations sustain glioblastoma cell dedifferentiation to a stem-like phenotype through survivin: possible involvment in radioresistance. Cell Death Dis. doi:10.1038/cddis.2014.509
Dawson A, Hillen T (2006) Derivation of the tumour control probability (TCP) for a cell cycle model. Comput Math Meth Med 7:121–142
de Almeida Sassi F, Brunetto AL, Schwartsmann G et al (2012) Glioma revisited: from neurogenesis and cancer stem cells to the epigenetic regulation of the niche. J Oncol 2012:537861
Dohi T, Beltrami E, Wall NR et al (2004) Mitochondrial survivin inhibits apoptosis and promotes tumorigenesis. J Clin Investig 114(8):1117–1127
Enderling H (2015) Cancer stem cells: small subpopulation or evolving fraction. Integr Biol 7(1):14–23
Eramo A, Haas TL, De Maria R (2010) Lung cancer stem cells: tools and targets to fight lung cancer. Oncogene 29(33):4625–4635
Fowler JF (2010) 21 Years of biologically effective dose. Br J Radiol 83(991):554–568
Gomez-Casal R, Bhattacharya C, Ganesh N et al (2013) Non-small cell lung cancer cells survived ionizing radiation treatment display cancer stem cell and epithelial-mesenchymal transition phenotypes. Mol Cancer. doi:10.1186/1476-4598-12-94
Gong J, dos Santos MM, Finlay C, Hillen T (2011) Are more complicated tumor control probability models better? Math Med Biol 30(1):1–19
Gupta PB, Fillmore CM, Jiang G et al (2011) Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cells 146(4):633–644
Guvenc H, Pavlyukov MS, Joshi K et al (2013) Impairment of glioma stem cell survival and growth by a novel inhibitor for survivin-ran protein complex. Clin Cancer Res 19(3):631–642
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
Hanin LG (2004) A stochastic model of tumor response to fractionated radiation: limit theorems and rate of convergence. Math Biosci 91(1):1–17
Hillen T, Enderling H, Hahnfeld P (2013) The tumor growth paradox and immune system-mediated selection for cancer stem cells. Bull Math Biol 75(1):161–184
Iwasa T, Okamoto I, Suzuki M et al (2008) Radiosensitizing effect of YM155, a novel small-molecule survivin suppressant, in non-small cell lung cancer cell lines. Clin Cancer Res 14(20):6496–6504
Jones L, Hoban P, Metcalfe P (2001) The use of the linear quadratic model in radiotherapy: a review. Australas Phys Eng Sci Med 24(3):132–146
Kang J, Zhang Y, Clump DA et al (2014) A free multi-model program for comparing linear-quadratic and non-linear quadratic models in TCP prediction of SABR-treated NSCLC. Int J Radiat Oncol Biol Phys 90(1):S854–S855
Klevebring D, Rosin G, Ma R et al (2014) Sequencing of breast cancer stem cell populations indicates a dynamic conversion between differentiation states in-vivo. Breast Cancer Res. doi:10.1186/bcr3687
Konstorum A, Hillen T, Lowengrub J (2016) Feedback regulation in a cancer stem cell model can cause an Allee effect. Bull Math Biol 78(4):754–785. doi:10.1007/s11538-016-0161-5
Lagadec C, Vlashi E, Donna LD et al (2012) Radiation-induced reprogramming of breast cancer cells. Stem Cells 30(5):833–844
Lapidot T, Sirard C, Vormoor J et al (1994) A cell initiating human acute myeloid leukaemia after transplantation into scid mice. Nature 367(6464):645–648
Lathia JD, Heddleston JM, Venere M et al (2011) Deadly teamwork: neural cancer stem cells and the microenvironment. Cell Stem Cell 8(5):482–485
Leder K, Pitter K, LaPlant Q et al (2014) Mathematical modeling of PDGF-driven glioblastoma reveals optimized radiation dosing schedules. Cell 156(3):603–616
Li C, Heidt DG, Dalerba P et al (2007) Identification of pancreatic cancer stem cells. Cancer Res 67(3):1030–1037
Maddalena L (2014) Analysis of an integro-differential system modeling tumor growth. Appl Math Comput 245:152–157
Marjanovic ND, Weinberg RA, Chaffer CL (2013) Cell plasticity and heterogeneity in cancer. Clin Chem 59(1):168–179
Nakahara T, Kita A, Yamanaka K et al (2007) YM155, a novel small-molecule survivin suppressant, induces regression of established human hormone-refractory prostate tumor xenografts. Cancer Res 67(17):8014–8021
Pennati M, Folini M, Zaffaroni N (2008) Targeting survivin in cancer therapy. Expert Opin Ther Targets 12(4):436–476
Phillips TM, McBride WH, Pajonk F (2006) The response of \(CD24^{-/low}\)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst 98(24):1777–1785
Poleszczuk J, Enderling H (2015) Cancer stem cell plasticity as tumor growth promoter and catalyst of population collapse. Stem Cells Int. Article ID: 713565
Rauch A, Hennig D, Schafer C et al (2014) Survivin and YM155: How faithful is the liaison? Biochim et Biophys Acta Rev Cancer 1845(2):202–220
Ricardi U, Badellino S, Filippi AR (2015) Stereotactic radiotherapy for early stage non small cell lung cancer. Radiat Oncol J 33(2):57–65
Sarvi S, Mackinnon AC, Avlonitis N et al (2014) CD133+ cancer stem-like cells in small cell lung cancer are highly tumorigenic and chemoresistant but sensitive to a novel neuropeptide antagonist. Cancer Res 74(5):1554–1565
Shuryak I, Carlson DJ, Brown M et al (2015) High-dose and fractionation effects in stereotactic radiation therapy: analysis of tumor control data from 2965 patients. Radiother Oncol 115(3):327–334
Singh SK, Clarke ID, Terasaki M et al (2003) Identification of a cancer stem cell in human brain tumors. Cancer Res 63(18):5821–5828
Sottoriva A, Sloot PMA, Medema JP et al (2010) Exploring cancer stem cell niche directed tumor growth. Cell Cycle 9(8):1472–1479
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4):663–676
Tirino V, Camerlingo R, Franco R et al (2009) The role of CD133 in the identification and characterisation of tumour-initiating cells in non-small-cell lung cancer. Eur J Cardiothorac Surg 36(3):446–453
Wang ZA, Hillen T (2007) Pattern formation for a chemotaxis model with volume filling effects. Chaos 17(3):037108
Yamanaka S (2009) Elite and stochastic models for induced pluripotent stem cell generation. Nature 460(7251):49–52
Youssefpour H, Li X, Lander A et al (2012) Multispecies model of cell lineages and feedback control in solid tumors. J Theor Biol 304:39–59
Yu VY, Nguyen D, Pajonk F et al (2015) Incorporating cancer stem cells in radiation therapy treatment response modeling and the implication in glioblastoma multiforme treatment resistance. Int J Radiat Oncol Biol Phys 91(4):866–875
Acknowledgments
AR is grateful to an NSERC USRA scholarship. TH appreciates support through an NSERC Discovery Grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rhodes, A., Hillen, T. Mathematical Modeling of the Role of Survivin on Dedifferentiation and Radioresistance in Cancer. Bull Math Biol 78, 1162–1188 (2016). https://doi.org/10.1007/s11538-016-0177-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-016-0177-x