Abstract
Background
Lung cancer harboring epidermal growth factor receptor (EGFR) mutations and treated with EGFR tyrosine kinase inhibitors (TKIs) all eventually develop acquired resistance to the treatment, with half of the patients developing EGFR T790M resistance mutations.
Objective
The purpose of this study was to assess histological and clinical characteristics and survival outcomes in Hispanic EGFR mutated lung cancer patients after disease progression.
Patients and Methods
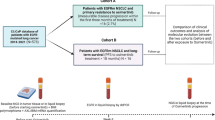
EGFR mutation-positive lung cancer patients (n = 34) with acquired resistance to the EGFR-TKI erlotinib were identified from 2011 to 2015. Post-progression tumor specimens were collected for molecular analysis. Post-progression interventions, response to treatment, and survival were assessed and compared among all patients and those with and without T790M mutations.
Results
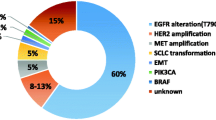
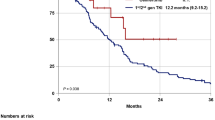
Mean age was 59.4 ± 13.9 years, 65% were never-smokers, and 53% had a performance status 0–1. All patients received erlotinib as first-line treatment. Identified mutations included: 60% DelE19 (Del746–750) and 40% L858R. First-line erlotinib overall response rate (ORR) was 61.8% and progression free survival (PFS) was 16.8 months (95% CI: 13.7–19.9). Acquired resistance mutations identified were T790M mutation (47.1%); PI3K mutations (14.7%); EGFR amplification (14.7%); KRAS mutation (5.9%); MET amplification (8.8%); HER2 alterations (5.9%, deletions/insertions in e20); and SCLC transformation (2.9%). Of patients, 79.4% received treatment after progression. ORR for post-erlotinib treatment was 47.1% (CR 2/PR 14) and median PFS was 8.3 months (95% CI: 2.2–36.6). Median overall survival (OS) from treatment initiation was 32.9 months (95% CI: 30.4–35.3), and only the use of post-progression therapy affected OS in a multivariate analysis (p = 0.05).
Conclusions
Hispanic patients with acquired resistance to erlotinib continued to be sensitive to other treatments after progression. The proportion of T790M+ patients appears to be similar to that previously reported in Caucasians.





Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11 Lyon, France. http://globocan.iarc.fr. Accessed 27 Aug 2016.
Brambilla E, Travis WD. Lung cancer. In: Stewart BW, Wild CP, editors. World cancer report. Lyon: World Health Organization; 2014.
Mitsudomi T, Yatabe Y. Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer. Cancer Sci. 2007;98(12):1817–24. doi:10.1111/j.1349-7006.2007.00607.x.
Sakurada A, Shepherd FA, Tsao MS. Epidermal growth factor receptor tyrosine kinase inhibitors in lung cancer: impact of primary or secondary mutations. Clin Lung Cancer. 2006;7(Suppl 4):S138–44.
Boolell V, Alamgeer M, Watkins DN, Ganju V. The evolution of therapies in non-small cell lung cancer. Cancers. 2015;7(3):1815–46. doi:10.3390/cancers7030864.
Arrieta O, Cardona AF, Martin C, Mas-Lopez L, Corrales-Rodriguez L, Bramuglia G, et al. Updated frequency of EGFR and KRAS mutations in NonSmall-cell lung cancer in Latin America: the Latin-American Consortium for the investigation of lung cancer (CLICaP). J Thorac Oncol. 2015;10(5):838–43. doi:10.1097/JTO.0000000000000481.
Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001;37(Suppl 4):S9–15.
Barron F, de la Torre-Vallejo M, Luna-Palencia RL, Cardona AF, Arrieta O. The safety of afatinib for the treatment of non-small cell lung cancer. Expert Opin Drug Saf. 2016;15(11):1563–72. doi:10.1080/14740338.2016.1236910.
Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–500. doi:10.1126/science.1099314.
Kobayashi S, Boggon TJ, Dayaram T, Janne PA, Kocher O, Meyerson M, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352(8):786–92. doi:10.1056/NEJMoa044238.
Greulich H, Chen TH, Feng W, Janne PA, Alvarez JV, Zappaterra M, et al. Oncogenic transformation by inhibitor-sensitive and -resistant EGFR mutants. PLoS Med. 2005;2(11):e313. doi:10.1371/journal.pmed.0020313.
Arrieta O, Cardona AF, Corrales L, Campos-Parra AD, Sanchez-Reyes R, Amieva-Rivera E, et al. The impact of common and rare EGFR mutations in response to EGFR tyrosine kinase inhibitors and platinum-based chemotherapy in patients with non-small cell lung cancer. Lung Cancer. 2015;87(2):169–75. doi:10.1016/j.lungcan.2014.12.009.
Li Z, Yu Y, Lu J, Luo Q, Wu C, Liao M, et al. Analysis of the T descriptors and other prognosis factors in pathologic stage I non-small cell lung cancer in China. J Thorac Oncol. 2009;4(6):702–9. doi:10.1097/JTO.0b013e3181a5269d.
Wang CL, Yue DS, Zhang ZF, Gong LQ, Su YJ, You J, et al. Treatment and prognostic analysis of 1638 patients with non-small cell lung cancer. Zhonghua Wai Ke Za Zhi [Chin J Surg]. 2011;49(7):618–22. doi:10.3760/cma.j.issn.0529-5815.2011.07.011.
Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC lung cancer staging project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2(8):706–14. doi:10.1097/JTO.0b013e31812f3c1a.
Wheeler DL, Dunn EF, Harari PM. Understanding resistance to EGFR inhibitors-impact on future treatment strategies. Nat Rev Clin Oncol. 2010;7(9):493–507. doi:10.1038/nrclinonc.2010.97.
Bianco R, Troiani T, Tortora G, Ciardiello F. Intrinsic and acquired resistance to EGFR inhibitors in human cancer therapy. Endocr Relat Cancer. 2005;12(Suppl 1):S159–71. doi:10.1677/erc.1.00999.
Ribeiro Gomes J, Cruz MR. Combination of afatinib with cetuximab in patients with EGFR-mutant non-small-cell lung cancer resistant to EGFR inhibitors. Onco Targets Ther. 2015;8:1137–42. doi:10.2147/OTT.S75388.
Riely GJ, Yu HA. EGFR: the paradigm of an oncogene-driven lung cancer. Clin Cancer Res. 2015;21(10):2221–6. doi:10.1158/1078-0432.CCR-14-3154.
Hwang KE, Jung JW, Oh SJ, Park MJ, Shon YJ, Choi KH, et al. Transformation to small cell lung cancer as an acquired resistance mechanism in EGFR-mutant lung adenocarcinoma: a case report of complete response to etoposide and cisplatin. Tumori. 2015;101(3):e96–8. doi:10.5301/tj.5000276.
Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3(75):75ra26. doi:10.1126/scitranslmed.3002003.
Arrieta O, Ramirez-Tirado LA, Baez-Saldana R, Pena-Curiel O, Soca-Chafre G, Macedo-Perez EO. Different mutation profiles and clinical characteristics among Hispanic patients with non-small cell lung cancer could explain the “Hispanic paradox”. Lung Cancer. 2015;90(2):161–6. doi:10.1016/j.lungcan.2015.08.010.
Jackman D, Pao W, Riely GJ, Engelman JA, Kris MG, Janne PA, et al. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. J Clin Oncol. 2010;28(2):357–60. doi:10.1200/JCO.2009.24.7049.
Xu M, Xie Y, Ni S, Liu H. The latest therapeutic strategies after resistance to first generation epidermal growth factor receptor tyrosine kinase inhibitors (EGFR TKIs) in patients with non-small cell lung cancer (NSCLC). Ann Transl Med. 2015;3(7):96. doi:10.3978/j.issn.2305-5839.2015.03.60.
Lee JC, Jang SH, Lee KY, Kim YC. Treatment of non-small cell lung carcinoma after failure of epidermal growth factor receptor tyrosine kinase inhibitor. Cancer Res Treat. 2013;45(2):79–85. doi:10.4143/crt.2013.45.2.79.
Haber DA, Velculescu VE. Blood-based analyses of cancer: circulating tumor cells and circulating tumor DNA. Cancer Discov. 2014;4(6):650–61. doi:10.1158/2159-8290.CD-13-1014.
Ilie M, Hofman V, Long E, Bordone O, Selva E, Washetine K, et al. Current challenges for detection of circulating tumor cells and cell-free circulating nucleic acids, and their characterization in non-small cell lung carcinoma patients. What is the best blood substrate for personalized medicine? Ann Transl Med. 2014;2(11):107. doi:10.3978/j.issn.2305-5839.2014.08.11.
Chen Q, Quan Q, Ding L, Hong X, Zhou N, Liang Y, et al. Continuation of epidermal growth factor receptor tyrosine kinase inhibitor treatment prolongs disease control in non-small-cell lung cancers with acquired resistance to EGFR tyrosine kinase inhibitors. Oncotarget. 2015;6(28):24904–11. doi:10.18632/oncotarget.4570.
Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19(8):2240–7. doi:10.1158/1078-0432.CCR-12-2246.
Kawano O, Sasaki H, Endo K, Suzuki E, Haneda H, Yukiue H, et al. PIK3CA mutation status in Japanese lung cancer patients. Lung Cancer. 2006;54(2):209–15. doi:10.1016/j.lungcan.2006.07.006.
Mino-Kenudson M. Programmed cell death ligand-1 (PD-L1) expression by immunohistochemistry: could it be predictive and/or prognostic in non-small cell lung cancer? Cancer Biol Med. 2016;13(2):157–70. doi:10.20892/j.issn.2095-3941.2016.0009.
Chen N, Fang W, Zhan J, Hong S, Tang Y, Kang S, et al. Upregulation of PD-L1 by EGFR activation mediates the immune escape in EGFR-driven NSCLC: implication for optional immune targeted therapy for NSCLC patients with EGFR mutation. J Thorac Oncol. 2015;10(6):910–23. doi:10.1097/JTO.0000000000000500.
Lin C, Chen X, Li M, Liu J, Qi X, Yang W, et al. Programmed death-ligand 1 expression predicts tyrosine kinase inhibitor response and better prognosis in a cohort of patients with epidermal growth factor receptor mutation-positive lung adenocarcinoma. Clin Lung Cancer. 2015;16(5):e25–35. doi:10.1016/j.cllc.2015.02.002.
Zhang Y, Wang L, Li Y, Pan Y, Wang R, Hu H, et al. Protein expression of programmed death 1 ligand 1 and ligand 2 independently predict poor prognosis in surgically resected lung adenocarcinoma. Onco Targets Ther. 2014;7:567–73. doi:10.2147/OTT.S59959.
Handorf EA, McElligott S, Vachani A, Langer CJ, Bristol Demeter M, Armstrong K, et al. Cost effectiveness of personalized therapy for first-line treatment of stage IV and recurrent incurable adenocarcinoma of the lung. J Oncol Pract. 2012;8(5):267–74. doi:10.1200/JOP.2011.000502.
Arrieta O, Anaya P, Morales-Oyarvide V, Ramirez-Tirado LA, Polanco AC. Cost-effectiveness analysis of EGFR mutation testing in patients with non-small cell lung cancer (NSCLC) with gefitinib or carboplatin-paclitaxel. Eur J Health Econ: HEPAC: Health Econ Prev Care. 2016;17(7):855–63. doi:10.1007/s10198-015-0726-5.
Acknowledgements
We are grateful for the generous contribution from the Silberman and Buendía families for altruistically promoting the development of cancer research in Colombia.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Funding
Funding Supported by the Foundation for Clinical and Applied Cancer Research -FICMAC (Bogotá, Colombia) research grant 018–2014.
Conflict of Interest
Andrés F. Cardona has received consulting fees or honorarium, support for travel to meetings for the study, manuscript preparation or other purposes, and payment for lectures including service on speakers bureaus from Roche, Pfizer, Bristol-Meyers Squibb, Merck, MSD, and AstraZeneca. Noemí Reguart has received consulting fees or honorarium for advisory roles, payment for lectures including service on speaker bureaus, and has given expert testimony for Boehringer Ingelheim, Roche, AstraZeneca, Bristol-Myers Squibb, and Pfizer. Luis Corrales has participated in advisory boards organized by AstraZeneca and received honoraria from AstraZeneca for lectures in scientific meetings. Carlos Ortiz has received consulting fees or honorarium for advisory boards for Pfizer, Amgen, and Roche.
All other authors declare no conflict of interest.
Disclaimer
Preliminary results from this study were previously presented during the 2015 European Cancer Congress – ESMO (25 September 2015, Vienna, Austria), during the Latin American Lung Cancer Conference - LALCA (25–27 August 2016, Panamá City, Panamá), and during the 17th World Conference on Lung Cancer – IASLC (4–7 December 2016, Vienna, Austria).
Additional information
Latin American Consortium for the Study of Lung Cancer
Electronic supplementary material
ESM 1
(PDF 255 kb)
Rights and permissions
About this article
Cite this article
Cardona, A.F., Arrieta, O., Zapata, M.I. et al. Acquired Resistance to Erlotinib in EGFR Mutation-Positive Lung Adenocarcinoma among Hispanics (CLICaP). Targ Oncol 12, 513–523 (2017). https://doi.org/10.1007/s11523-017-0497-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-017-0497-2