Abstract
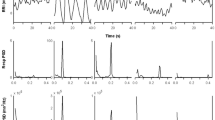
This work investigates the performance of cardiorespiratory analysis detecting periodic breathing (PB) in chest wall recordings in mountaineers climbing to extreme altitude. The breathing patterns of 34 mountaineers were monitored unobtrusively by inductance plethysmography, ECG and pulse oximetry using a portable recorder during climbs at altitudes between 4497 and 7546 m on Mt. Muztagh Ata. The minute ventilation (VE) and heart rate (HR) signals were studied, to identify visually scored PB, applying time-varying spectral, coherence and entropy analysis. In 411 climbing periods, 30–120 min in duration, high values of mean power (MPVE) and slope (MSlopeVE) of the modulation frequency band of VE, accurately identified PB, with an area under the ROC curve of 88 and 89 %, respectively. Prolonged stay at altitude was associated with an increase in PB. During PB episodes, higher peak power of ventilatory (MPVE) and cardiac (MP HRLF ) oscillations and cardiorespiratory coherence (MP CoherLF ), but reduced ventilation entropy (SampEnVE), was observed. Therefore, the characterization of cardiorespiratory dynamics by the analysis of VE and HR signals accurately identifies PB and effects of altitude acclimatization, providing promising tools for investigating physiologic effects of environmental exposures and diseases.






Similar content being viewed by others
References
Bloch KE, Barandun J, Sackner MA (1995) Effect of mouthpiece breathing on cardiorespiratory response to intense exercise. Am J Respir Crit Care Med 151:1087–1092
Bloch K, Myers J, Senn O (2006) Sample entropy as a metric to quantify periodic breathing during high altitude climbing. Eur Respir J 28(suppl):195s
Bloch KE, Turk AJ, Maggiorini M et al (2009) Effect of ascent protocol on acute mountain sickness and success at Muztagh Ata, 7546 m. High Alt Med Biol 10:25–32
Bloch KE, Latshang TD, Turk AJ et al (2010) Nocturnal periodic breathing during acclimatization at very high altitude at Mount Muztagh Ata (7,546 m). Am J Respir Crit Care Med 182:562–568
Brack T (2003) Cheyne-Stokes respiration in patients with congestive heart failure. Swiss Med Wkly 133:605–610
Brack T, Thüer I, Clarenbach CFC et al (2007) Daytime Cheyne-Stokes respiration in ambulatory patients with severe congestive heart failure is associated with increased mortality. Chest 132:1463–1471
Brüllmann G, Thurnheer R, Bloch E et al (2010) Respiratory monitoring by inductive plethysmography in unrestrained subjects using position sensor-adjusted calibration. Respiration 79:112–120
Chen X, Solomon I, Chon K (2005) Comparison of the use of approximate entropy and sample entropy: applications to neural respiratory signal. Proc IEEE Conf Eng Med Biol 4:4212–4215
Clarenbach CF, Senn O, Bloch KE (2003) A portable inductance plethysmograph for monitoring ventilation in unrestrained subjects. Eur Respir J 22:408s
Dempsey JA (2005) Crossing the apnoeic threshold: causes and consequences. Exp Physiol 90:13–24
Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP (2010) Pathophysiology of sleep apnea. Physiol Rev 90:47–112
Fowler AC (2003) Periodic breathing at high altitude. J Math Appl Med Biol 19:293–313
Gallagher S, Hackett PH (2004) High-altitude illness. Emerg Med Clin N Am 22:329–355, viii
Garde A, Giraldo BF, Jané R, Sornmo L (2009) Time-varying respiratory pattern characterization in chronic heart failure patients and healthy subjects. Proc. IEEE Conf. Eng. Med. Biol. Minneapolis, Minnesota, USA, pp 4007–4010
Garde A, Sörnmo L, Jané R, Giraldo BF (2010) Breathing pattern characterization in chronic heart failure patients using the respiratory flow signal. Ann Biomed Eng 38:3572–3580
Garde A, Sörnmo L, Jané R, Giraldo BF (2010) Correntropy-based spectral characterization of respiratory patterns in patients with chronic heart failure. IEEE Trans Biomed Eng 57:1964–1972
Garde A, Sornmo L, Jane R, Giraldo BF (2010) Correntropy-based nonlinearity test applied to patients with chronic heart failure. Proc IEEE Conf Eng Med Biol 2010:2399–2402
Garde A, Giraldo BF, Jane R et al (2012) Periodic breathing during ascent to extreme altitude quantified by spectral analysis of the respiratory volume signal. Proc IEEE Conf Eng Med Biol 2012:707–710
Indic P, Bloch-Salisbury E, Bednarek F et al (2011) Assessment of cardio-respiratory interactions in preterm infants by bivariate autoregressive modeling and surrogate data analysis. Early Hum Dev 87:477–487
Khoo MC, Kronauer RE, Strohl KP, Slutsky AS (1982) Factors inducing periodic breathing in humans: a general model. J Appl Physiol 53:644–659
Kohler M, Kriemler S, Wilhelm EM et al (2008) Children at high altitude have less nocturnal periodic breathing than adults. Eur Respir J 32:189–197
Latshang TD, Turk J, Hess T et al (2013) Acclimatization improves submaximal exercise economy at 5533 m. Scand J Med Sci Sports 23(4):458–467
Latshang TD, Cascio CM, Lo GrimmM et al (2013) Are nocturnal breathing, sleep and cognitive performance impaired at moderate altitude (1630–2590 m)? Sleep 36:1969–1976
Lipsitz LA, Hashimoto F, Lubowsky LP et al (1995) Heart rate and respiratory rhythm dynamics on ascent to high altitude. Br Heart J 74:390–396
Malik M, Bigger JT, Camm AJ et al (1996) Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Hear J 17:354–381
Mortara A, Sleight P, Pinna GD et al (1997) Abnormal awake respiratory patterns are common in chronic heart failure and may prevent evaluation of autonomic tone by measures of heart rate variability. Circulation 96:246–252
Nussbaumer-Ochsner Y, Schuepfer N, Siebenmann C, Maggiorini MBK (2011) High altitude sleep disturbances monitored by actigraphy and polysomnography. High Alt Med Biol 12(3):229–236
Nussbaumer-Ochsner Y, Ursprung J, Siebenmann C et al (2012) Effect of short-term acclimatization to high altitude on sleep and nocturnal breathing. Sleep 35:419–423
Pincus SM, Goldberger AL (1994) Physiological time-series analysis: what does regularity quantify? Am J Physiol Hear Circ Physiol 266:H1643–H1656
Rissanen J (1978) Modeling by shortest data description. Automatica 14:465–471
Sackner MA, Watson H, Belsito AS et al (1989) Calibration of respiratory inductive plethysmograph during natural breathing. J Appl Physiol 66:410–420
San T, Polat S, Cingi C et al (2013) Effects of high altitude on sleep and respiratory system and theirs adaptations. Sci World J 2013:241569. doi:10.1155/2013/241569
Sleep Medicine Task Force AA (1999) Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep 22:667–689
Sörnmo L, Laguna P (2005) Bioelectrical signal processing in cardiac and neurological applications. Elsevier/Academic Press, Amsterdam
Szollosi I, Krum H, Kaye D, Naughton MT (2007) Sleep apnea in heart failure increases heart rate variability and sympathetic dominance. Sleep 11:1509–1514
White LB, Boashash B (1990) Cross spectral analysis of nonstationary processes. IEEE Trans Inf Theory 36:830–835
Wolf MB, Garner RP (2009) Disequilibrium between alveolar and end-pulmonary-capillary O2 tension in altitude hypoxia and respiratory disease: an update of a mathematical model of human respiration at altitude. Ann Biomed Eng 37:1818–1826
Nussbaumer-Ochsner Y, Bloch KE (2014) Sleep. In: Swenson ER, Bärtsch P (eds) High altitude. Human adaptation to hypoxia. Springer, New York, pp 325–335
Acknowledgments
This work was supported by an international cooperation Grant of the Swiss National Science Foundation (SNSF), a mobility grant of the CIBER de Bioingeniería, Biomateriales y Nanomedicina (CIBER-BBN) and by the Ministerio de Economía y Competitividad from Spanish Government under Grant TEC2010-21703-C03-01, and by Grants from the Lung Ligue of Zurich, Switzerland.
Author information
Authors and Affiliations
Corresponding author
Appendix: Methods used for time series analysis
Appendix: Methods used for time series analysis
1.1 Power spectral density
The signal x(n) is modeled through an autoregressive model by
where e(n) denotes zero-mean white noise with variance σ 2 e , a[k] the AR coefficients and p the model order. Once the autoregressive coefficients and the variance σ 2 e have been estimated, the PSD of an autoregressive process is computed by means of
being T the sampling period.
The advantage of model-based frequency estimation is its capacity to predict future samples outside of the observation interval, instead of assuming zero as occurs with conventional nonparametric or Fourier-based spectral analysis [34]. The accuracy of the AR model was evaluated through the mean square prediction error. The optimum model order (ranging from 2 to 50) was selected for each signal according to the criterion proposed by Rissanen [30], based on selecting the model order that minimizes the description length.
1.2 Coherence
The coherence function measures the strength of the linear interaction between two time series at each frequency. Its value ranges from 0, implying no temporal correlation between the two signals to 1 implying maximum correlation. It is described as
where P x (f) and P y (f) are the PSD of both signals x(n) and y(n), respectively, and P xy (f) is the cross-PSD between them. In order to get higher frequency resolution, a parametric coherence is implemented in this study [19].
In parametric coherence analysis, a bivariate autoregressive model is applied to calculate the parametric cross-PSD. The relationship between both signals is described by
where the matrix S contains both signals, matrix A autoregressive coefficients and matrix E zero-mean white noise inputs.
The matrix of PSD for this bivariate autoregressive model is obtained by
where * denotes the conjugate transpose of the matrix. The matrix A is obtained through
1.3 Complexity analysis
Approximate entropy (ApEn) and sample entropy (SampEn) provide quantitative information about the complexity of the signals. ApEn is approximately equal to the negative average natural logarithm of the conditional probability that two sequences that are similar for m points remain similar, that is, within a tolerance r, at the next point. In order to avoid the occurrence of ln(0) in the calculations, ApEn algorithm counts each sequence as matching itself. ApEn is therefore heavily dependent on the record length and lacks relative consistency. SampEn is the negative natural logarithm of the conditional probability that two sequences similar for m points remain similar at the next point, where self-matches are not included in calculating the probability [8].
Considering the signal x(n) and the parameters m and r, the ApEn is computed by
-
1.
Form the m-vectors
$$\varvec{X}(i) = \left[ {x(i), x(i + 1), \ldots ,x\left( {i + m - 1} \right)} \right];\quad 1 \le i \le N - m + 1$$being N the length of the signal.
-
2.
Calculate distances between vectors
$$d[X(i),X(j)] = \max_{k = 1, \ldots m} \left( {\left| {x\left( {i + k} \right) - x(j + k)} \right|} \right)$$ -
3.
Compute frequency or regularity of similar patterns
$$C_{r}^{m} (i) = \frac{{N^{m} (i)}}{N - m + 1}$$where N m(i) is the number of \(j \left( {1 \le j \le N - m + 1} \right)\) so that \(d\left[ {X(i),X(j)} \right] \le r.\)
-
4.
Take the natural logarithm of each C m r and average it over
$$\emptyset^{m} (r) = \frac{1}{N - m + 1}\sum\limits_{i = 1}^{N - m + 1} {\ln \left( {C_{r}^{m} (i)} \right)}$$ -
5.
Repeat this process for m + 1 and the ApEn is finally computed as follows
$$ApEn\left( {m,r,N} \right) = \emptyset^{m} (r) - \emptyset^{m + 1} (r)$$
Considering the signal x(n) and the parameters m and r, the SampEn is computed by
-
1.
Form the m-vectors
$$\varvec{X}(i) = \left[ {x(i), x(i + 1), \ldots ,x(i + m - 1)} \right];\quad 1 \le i \le N - m + 1$$being N the length of the signal.
-
2.
Calculate distances between vectors
$$d[X(i),X(j)] = \max_{k = 1, \ldots m} \left( {\left| {x\left( {i + k} \right) - x(j + k)} \right|} \right)$$ -
3.
Define for each of \(i\left( {1 \le i \le N - m } \right)\)
$$\begin{aligned} B_{i}^{m} (r) & = \frac{{N^{m} (i)}}{N - m + 1} \\ A_{i}^{m} (r) & = \frac{{N^{m + 1} (i)}}{N - m + 1} \\ \end{aligned}$$where N m(i) is the number of \(j \left( {1 \le j \le N - m + 1} \right),\;j \ne i\) so that \(d\left[ {X(i),X(j)} \right]_{m} \le r,\) and N m+1(i) is the number of \(j\left( {1 \le j \le N - m + 1} \right),\;j \ne i\) so that \(d\left[ {X(i),X(j)} \right]_{m + 1} \le r\).
-
4.
Compute
$$\begin{aligned} B^{m} (r) & = \frac{1}{N - m }\sum\limits_{i = 1}^{N - m} {B_{i}^{m} (r)} \\ A^{m} (r) & = \frac{1}{N - m }\sum\limits_{i = 1}^{N - m} {A_{i}^{m} (r)} \\ \end{aligned}$$ -
5.
SampEn is finally computed as follows
$$SampEn\left( {m,r,N} \right) = - \ln (A^{m} (r)/B^{m} (r))$$
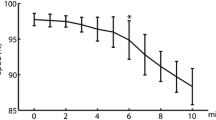
The complexity of the VE and HR signals was evaluated with tolerance values ranging from 0.05 to 0.5 and m = 2 (see Fig. 7). Sample entropy and approximate entropy of VE and HR for PB and nPB periods with different tolerance values are plotted. According to this analysis and previous studies, the tolerance value of 0.15 was selected for further processing [2].
Rights and permissions
About this article
Cite this article
Garde, A., Giraldo, B.F., Jané, R. et al. Time-varying signal analysis to detect high-altitude periodic breathing in climbers ascending to extreme altitude. Med Biol Eng Comput 53, 699–712 (2015). https://doi.org/10.1007/s11517-015-1275-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-015-1275-x