Abstract
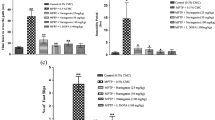
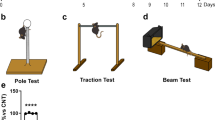
Increasing the function of residual dopaminergic neurons in the nigra of PD patients is an important area of research as it may eventually compensate the loss. Although tyrosine hydroxylase (TH) is the rate-limiting enzyme in the dopamine (DA) biosynthesis pathway, there are no effective drugs/molecules to upregulate TH and increase the production of DA in nigral dopaminergic neurons. This study underlines the importance of aspirin in stimulating the expression of TH and increasing the level of DA in dopaminergic neurons. At low doses, aspirin increased the expression of TH and the production of DA in mouse MN9D dopaminergic neuronal cells. Accordingly, oral administration of aspirin increased the expression of TH in the nigra and upregulated the level of DA in striatum of normal C57/BL6 mice and aged A53T α-syn transgenic mice. Oral aspirin also improved locomotor activities of normal mice and A53T transgenic mice. While investigating mechanisms, we found the presence of cAMP response element (CRE) in the promoter of TH gene and the rapid induction of cAMP response element binding (CREB) activation by aspirin in dopaminergic neuronal cells. Aspirin treatment also increased the level of phospho-CREB in the nigra of C57/BL6 mice. The abrogation of aspirin-induced expression of TH by siRNA knockdown of CREB and the recruitment of CREB to the TH gene promoter by aspirin suggest that aspirin stimulates the transcription of TH in dopaminergic neurons via CREB. These results highlight a new property of aspirin in stimulating the TH-DA pathway, which may be beneficial in PD patients.

ᅟ









Similar content being viewed by others
References
Aubin N, Curet O, Deffois A, Carter C (1998) Aspirin and salicylate protect against MPTP-induced dopamine depletion in mice. J Neurochem 71:1635–1642
Belgacem YH, Borodinsky LN (2017) CREB at the crossroads of activity-dependent regulation of nervous system development and function. Adv Exp Med Biol 1015:19–39
Benner EJ, Mosley RL, Destache CJ, Lewis TB, Jackson-Lewis V, Gorantla S, Nemachek C, Green SR, Przedborski S, Gendelman HE (2004) Therapeutic immunization protects dopaminergic neurons in a mouse model of Parkinson's disease. Proc Natl Acad Sci U S A 101:9435–9440
Berk M, Dean O, Drexhage H, McNeil JJ, Moylan S, O'Neil A, Davey CG, Sanna L, Maes M (2013) Aspirin: a review of its neurobiological properties and therapeutic potential for mental illness. BMC Med 11:74
Brahmachari S, Pahan K (2007) Sodium benzoate, a food additive and a metabolite of cinnamon, modifies T cells at multiple steps and inhibits adoptive transfer of experimental allergic encephalomyelitis. J Immunol 179:275–283
Brahmachari S, Jana A, Pahan K (2009) Sodium benzoate, a metabolite of cinnamon and a food additive, reduces microglial and astroglial inflammatory responses. J Immunol 183:5917–5927
Chandra G, Kundu M, Rangasamy SB, Dasarathy S, Ghosh S, Watson R, Pahan K (2017) Increase in Mitochondrial Biogenesis in Neuronal Cells by RNS60, a Physically-Modified Saline, via Phosphatidylinositol 3-Kinase-Mediated Upregulation of PGC1alpha. J NeuroImmune Pharmacol
Chandra S, Jana M, Pahan K (2018) Aspirin induces Lysosomal biogenesis and attenuates amyloid plaque pathology in a mouse model of Alzheimer's disease via PPARalpha. J Neurosci 38:6682–6699
Corbett GT, Roy A, Pahan K (2012) Gemfibrozil, a lipid-lowering drug, upregulates IL-1 receptor antagonist in mouse cortical neurons: implications for neuronal self-defense. J Immunol 189:1002–1013
Dai Y, Ge J (2012) Clinical use of aspirin in treatment and prevention of cardiovascular disease. Thrombosis 2012:245037
Dasgupta S, Jana M, Liu X, Pahan K (2003) Role of very-late antigen-4 (VLA-4) in myelin basic protein-primed T cell contact-induced expression of proinflammatory cytokines in microglial cells. J Biol Chem 278:22424–22431
Dauer W, Przedborski S (2003) Parkinson's disease: mechanisms and models. Neuron 39:889–909
Gagne JJ, Power MC (2010) Anti-inflammatory drugs and risk of Parkinson disease: a meta-analysis. Neurology 74:995–1002
Ghosh A, Pahan K (2012) Gemfibrozil, a lipid-lowering drug, induces suppressor of cytokine signaling 3 in glial cells: implications for neurodegenerative disorders. J Biol Chem 287:27189–27203
Ghosh A, Roy A, Liu X, Kordower JH, Mufson EJ, Hartley DM, Ghosh S, Mosley RL, Gendelman HE, Pahan K (2007) Selective inhibition of NF-kappaB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson's disease. Proc Natl Acad Sci U S A 104:18754–18759
Jana A, Modi KK, Roy A, Anderson JA, van Breemen RB, Pahan K (2013) Up-regulation of neurotrophic factors by cinnamon and its metabolite sodium benzoate: therapeutic implications for neurodegenerative disorders. J NeuroImmune Pharmacol 8:739–755
Jana M, Ghosh S, Pahan K (2018) Upregulation of myelin gene expression by a physically-modified saline via phosphatidylinositol 3-kinase-mediated activation of CREB: implications for multiple sclerosis. Neurochem Res 43:407–419
Kern S, Skoog I, Ostling S, Kern J, Borjesson-Hanson A (2012) Does low-dose acetylsalicylic acid prevent cognitive decline in women with high cardiovascular risk? A 5-year follow-up of a non-demented population-based cohort of Swedish elderly women. BMJ Open 2:e001288
Khasnavis S, Pahan K (2012) Sodium benzoate, a metabolite of cinnamon and a food additive, upregulates neuroprotective Parkinson disease protein DJ-1 in astrocytes and neurons. J NeuroImmune Pharmacol 7:424–435
Khasnavis S, Pahan K (2014) Cinnamon treatment upregulates neuroprotective proteins Parkin and DJ-1 and protects dopaminergic neurons in a mouse model of Parkinson's disease. J NeuroImmune Pharmacol 9:569–581
Khasnavis S, Jana A, Roy A, Mazumder M, Bhushan B, Wood T, Ghosh S, Watson R, Pahan K (2012) Suppression of nuclear factor-kappaB activation and inflammation in microglia by physically modified saline. J Biol Chem 287:29529–29542
Khasnavis S, Roy A, Ghosh S, Watson R, Pahan K (2014) Protection of dopaminergic neurons in a mouse model of Parkinson's disease by a physically-modified saline containing charge-stabilized nanobubbles. J NeuroImmune Pharmacol 9:218–232
Kopp E, Ghosh S (1994) Inhibition of NF-kappa B by sodium salicylate and aspirin. Science 265:956–959
Lang AE, Lozano AM (1998) Parkinson's disease. First of two parts. N Engl J Med 339:1044–1053
Marsden CD (1990) Parkinson's disease. Lancet 335:948–952
Modi KK, Sendtner M, Pahan K (2013) Up-regulation of ciliary neurotrophic factor in astrocytes by aspirin: implications for remyelination in multiple sclerosis. J Biol Chem 288:18533–18545
Modi KK, Rangasamy SB, Dasarathi S, Roy A, Pahan K (2016) Cinnamon converts poor learning mice to good learners: implications for memory improvement. J NeuroImmune Pharmacol 11:693–707
Mondal S, Roy A, Pahan K (2009) Functional blocking monoclonal antibodies against IL-12p40 homodimer inhibit adoptive transfer of experimental allergic encephalomyelitis. J Immunol 182:5013–5023
Mondal S, Roy A, Jana A, Ghosh S, Kordower JH, Pahan K (2012) Testing NF-kappaB-based therapy in hemiparkinsonian monkeys. J NeuroImmune Pharmacol 7:544–556
Moyad MA (2001) An introduction to aspirin, NSAids, and COX-2 inhibitors for the primary prevention of cardiovascular events and cancer and their potential preventive role in bladder carcinogenesis: part II. Semin Urol Oncol 19:306–316
Nilsson SE, Johansson B, Takkinen S, Berg S, Zarit S, McClearn G, Melander A (2003) Does aspirin protect against Alzheimer's dementia? A study in a Swedish population-based sample aged > or =80 years. Eur J Clin Pharmacol 59:313–319
Olanow CW, Tatton WG (1999) Etiology and pathogenesis of Parkinson's disease. Annu Rev Neurosci 22:123–144
Patel D, Roy A, Kundu M, Jana M, Luan CH, Gonzalez FJ, Pahan K (2018) Aspirin binds to PPARalpha to stimulate hippocampal plasticity and protect memory. Proc Natl Acad Sci U S A 115:E7408–E7417
Ross GW, Petrovitch H, Abbott RD, Nelson J, Markesbery W, Davis D, Hardman J, Launer L, Masaki K, Tanner CM, White LR (2004) Parkinsonian signs and substantia nigra neuron density in decendents elders without PD. Ann Neurol 56:532–539
Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z (2012) Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet 379:1591–1601
Roy A, Pahan K (2013) Myelin basic protein-primed T helper 2 cells suppress microglial activation via AlphaVBeta3 integrin: implications for multiple sclerosis. J Clin Cell Immunol 7:158
Roy A, Pahan K (2015) PPARalpha signaling in the hippocampus: crosstalk between fat and memory. J NeuroImmune Pharmacol 10:30–34
Roy A, Fung YK, Liu X, Pahan K (2006) Up-regulation of microglial CD11b expression by nitric oxide. J Biol Chem 281:14971–14980
Roy A, Jana M, Kundu M, Corbett GT, Rangaswamy SB, Mishra RK, Luan CH, Gonzalez FJ, Pahan K (2015) HMG-CoA Reductase inhibitors bind to PPARalpha to Upregulate Neurotrophin expression in the brain and improve memory in mice. Cell Metab 22:253–265
Saura CA, Cardinaux JR (2017) Emerging roles of CREB-regulated transcription Coactivators in brain physiology and pathology. Trends Neurosci 40:720–733
Surmeier DJ, Obeso JA, Halliday GM (2017) Selective neuronal vulnerability in Parkinson disease. Nat Rev Neurosci 18:101–113
Vane JR (1971) Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol 231:232–235
Vane JR, Botting RM (2003) The mechanism of action of aspirin. Thromb Res 110:255–258
Vila M, Przedborski S (2004) Genetic clues to the pathogenesis of Parkinson's disease. Nat Med 10(Suppl):S58–S62
WHO (2017) 20th Model List of Essential Medicine. In: http://www.who.int/medicines/publications/essentialmedicines/20th_EML2017_FINAL_amendedAug2017.pdf?ua=1, pp 2,25,36,50
Zhang F, Lu YF, Wu Q, Liu J, Shi JS (2012) Resveratrol promotes neurotrophic factor release from astroglia. Exp Biol Med (Maywood) 237:943–948
Acknowledgements
This study was supported by merit awards from Department of Veteran Affairs (I01BX002174 and I01BX003033) and a grant (NS083054) from NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
None.
Electronic supplementary material
ESM 1
(PDF 1105 kb)
Rights and permissions
About this article
Cite this article
Rangasamy, S.B., Dasarathi, S., Pahan, P. et al. Low-Dose Aspirin Upregulates Tyrosine Hydroxylase and Increases Dopamine Production in Dopaminergic Neurons: Implications for Parkinson’s Disease. J Neuroimmune Pharmacol 14, 173–187 (2019). https://doi.org/10.1007/s11481-018-9808-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-018-9808-3