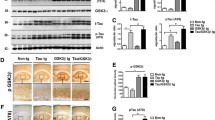
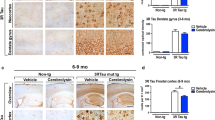
Abstract
Because of their neuroprotective properties, cannabinoids are being investigated in neurodegenerative disorders, mainly in preclinical studies. These disorders also include amyotrophic lateral sclerosis (ALS), a degenerative disease produced by the damage of the upper and lower motor neurons leading to muscle denervation, atrophy and paralysis. The studies with cannabinoids in ALS have been conducted exclusively in a transgenic mouse model bearing mutated forms of human superoxide dismutase-1, the first gene that was identified in relation with ALS. The present study represents the first attempt to investigate the endocannabinoid system in an alternative model, the transgenic mouse model of TAR-DNA binding protein-43 (TDP-43), a protein related to ALS and also to frontotemporal dementia. We used these mice for behavioral and histological characterization at an early symptomatic phase (70–80 days of age) and at a post-symptomatic stage (100–110 days of age). TDP-43 transgenic mice exhibited a worsened rotarod performance at both disease stages. This was accompanied by a loss of motor neurons in the spinal cord (measured by Nissl staining) and by reactive microgliosis (measured by Iba-1 immunostaining) at the post-symptomatic stage. We also detected elevated levels of the CB2 receptor (measured by qRT-PCR and western blotting) in the spinal cord of these animals. Double-staining studies confirmed that this up-regulation occurs in microglial cells in the post-symptomatic stage. Some trends towards an increase were noted also for the levels of endocannabinoids, which in part correlate with a small reduction of FAAH. Some of these parameters were also analyzed in the cerebral cortex of TDP-43 transgenic mice, but we did not observe any significant change, in agreement with the absence of anomalies in cognitive tests. In conclusion, our data support the idea that the endocannabinoid signaling system, in particular the CB2 receptor, may serve for the development of a neuroprotective therapy in TDP-43-related disorders. We are presently engaged in pharmacological experiments to investigate this possibility.







Similar content being viewed by others
References
Al-Chalabi A, Hardiman O (2013) The epidemiology of ALS: a conspiracy of genes, environment and time. Nat Rev Neurol 9:617–628
Alvarez FJ, Lafuente H, Rey-Santano MC et al (2008) Neuroprotective effects of the nonpsychoactive cannabinoid cannabidiol in hypoxic-ischemic newborn piglets. Pediatr Res 64:653–658
Atwood BK, Mackie K (2010) CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol 160:467–479
Bilsland LG, Dick JR, Pryce G et al (2006) Increasing cannabinoid levels by pharmacological and genetic manipulation delay disease progression in SOD1 mice. FASEB J 20:1003–1005
Bisogno T, Sepe N, Melck D et al (1997) Biosynthesis, release and degradation of the novel endogenous cannabimimetic metabolite 2-arachidonoylglycerol in mouse neuroblastoma cells. Biochem J 322:671–677
Buratti E, Baralle FE (2010) The multiple roles of TDP-43 in pre-mRNA processing and gene expression regulation. RNA Biol 7:420–429
Cruts M, Gijselinck I, Van Langenhove T, van der Zee J, Van Broeckhoven C (2013) Current insights into the C9orf72 repeat expansion diseases of the FTLD/ALS spectrum. Trends Neurosci 36:450–459
de Lago E, Moreno-Martet M, Espejo-Porras F, Fernández-Ruiz J (2015) Endocannabinoids and amyotrophic lateral sclerosis. In: Fattore L (ed) Cannabinoids in neurologic and mental disease. Elsevier, The Netherlands, pp 99–124
Devane WA, Hanus L, Breuer A et al (1992) Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258:1946–1949
Esmaeili MA, Panahi M, Yadav S, Hennings L, Kiaei M (2013) Premature death of TDP-43 (A315T) transgenic mice due to gastrointestinal complications prior to development of full neurological symptoms of amyotrophic lateral sclerosis. Int J Exp Pathol 94:56–64
Fernández-Ruiz J, Romero J, Velasco G et al (2007) Cannabinoid CB2 receptor: a new target for controlling neural cell survival? Trends Pharmacol Sci 28:39–45
Fernández-Ruiz J, García C, Sagredo O, Gómez-Ruiz M, de Lago E (2010) The endocannabinoid system as a target for the treatment of neuronal damage. Expert Opin Ther Targets 14:387–404
Ferraiuolo L, Kirby J, Grierson AJ, Sendtner M, Shaw PJ (2011) Molecular pathways of motor neuron injury in amyotrophic lateral sclerosis. Nat Rev Neurol 7:616–630
Foran E, Trotti D (2009) Glutamate transporters and the excitotoxic path to motor neuron degeneration in amyotrophic lateral sclerosis. Antioxid Redox Signal 11:1587–1602
Guo Y, Wang Q, Zhang K, An T, Shi P, Li Z, Duan W, Li C (2012) HO-1 induction in motor cortex and intestinal dysfunction in TDP-43 A315T transgenic mice. Brain Res 1460:88–95
Gürtler A, Kunz N, Gomolka M et al (2013) Stain-Free technology as a normalization tool in Western blot analysis. Anal Biochem 433:105–111
Habib AA, Mitsumoto H (2011) Emerging drugs for amyotrophic lateral sclerosis. Expert Opin Emerg Drugs 16:537–558
Hardiman O, van den Berg LH, Kiernan MC (2011) Clinical diagnosis and management of amyotrophic lateral sclerosis. Nat Rev Neurol 7:639–649
Janssens J, Van Broeckhoven C (2013) Pathological mechanisms underlying TDP-43 driven neurodegeneration in FTLD-ALS spectrum disorders. Hum Mol Genet 22:R77–R87
Kim K, Moore DH, Makriyannis A, Abood ME (2006) AM1241, a cannabinoid CB2 receptor selective compound, delays disease progression in a mouse model of amyotrophic lateral sclerosis. Eur J Pharmacol 542:100–105
Lagier-Tourenne C, Polymenidou M, Cleveland DW (2010) TDP-43 and FUS/TLS: emerging roles in RNA processing and neurodegeneration. Hum Mol Genet 19:R46–R64
Marsicano G, Wotjak CT, Azad SC et al (2002) The endogenous cannabinoid system controls extinction of aversive memories. Nature 418:530–534
Moreno-Martet M, Espejo-Porras F, Fernández-Ruiz J, de Lago E (2014) Changes in endocannabinoid receptors and enzymes in the spinal cord of SOD1(G93A) transgenic mice and evaluation of a Sativex®-like combination of phytocannabinoids: interest for future therapies in amyotrophic lateral sclerosis. CNS Neurosci Ther 20:809–815
Patil SS, Sunyer B, Höger H, Lubec G (2009) Evaluation of spatial memory of C57BL/6 J and CD1 mice in the Barnes maze, the Multiple T-maze and in the Morris water maze. Behav Brain Res 198:58–68
Raman C, McAllister SD, Rizvi G et al (2004) Amyotrophic lateral sclerosis: delayed disease progression in mice by treatment with a cannabinoid. Amyotroph Lateral Scler Other Motor Neuron Disord 5:33–39
Renton AE, Chiò A, Traynor BJ (2014) State of play in amyotrophic lateral sclerosis genetics. Nat Neurosci 17:17–23
Ripps ME, Huntley GW, Hof PR, Morrison JH, Gordon JW (1995) Transgenic mice expressing an altered murine superoxide dismutase gene provide an animal model of amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A 92:689–693
Robberecht W, Philips T (2013) The changing scene of amyotrophic lateral sclerosis. Nat Rev Neurosci 14:248–264
Rosen DR, Siddique T, Patterson D et al (1993) Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362:59–62
Shoemaker JL, Seely KA, Reed RL et al (2007) The CB2 cannabinoid agonist AM-1241 prolongs survival in a transgenic mouse model of amyotrophic lateral sclerosis when initiated at symptom onset. J Neurochem 101:87–98
Tsao W, Jeong YH, Lin S, Ling J, Price DL, Chiang PM, Wong PC (2012) Rodent models of TDP-43: recent advances. Brain Res 1462:26–39
Vázquez C, García MC, Tolón RM et al (2014) New mouse model for the study of CB2 cannabinoid receptors. The 24th Annual Symposium on the Cannabinoids. International Cannabinoid Research Society, Research Triangle Park, NC, USA, page 260
Weber M, Goldman B, Truniger S (2010) Tetrahydrocannabinol (THC) for cramps in amyotrophic lateral sclerosis: a randomised, double-blind crossover trial. J Neurol Neurosurg Psychiatry 81:1135–1140
Wegorzewska I, Bell S, Cairns NJ, Miller TM, Baloh RH (2009) TDP-43 mutant transgenic mice develop features of ALS and frontotemporal lobar degeneration. Proc Natl Acad Sci U S A 106:18809–18814
Weydt P, Hong S, Witting A et al (2005) Cannabinol delays symptom onset in SOD1 (G93A) transgenic mice without affecting survival. Amyotroph Lateral Scler Other Motor Neuron Disord 6:182–184
Witting A, Weydt P, Hong S et al (2004) Endocannabinoids accumulate in spinal cord of SOD1 G93A transgenic mice. J Neurochem 89:1555–1557
Yiangou Y, Facer P, Durrenberger P et al (2006) COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC Neurol 6:12
Acknowledgements
This work has been supported by grants from CIBERNED (CB06/05/0089), MINECO (SAF2012/39173), CAM (S2011/BMD-2308), Alzheimer’s Association USA and GW Pharmaceuticals Ltd. These agencies had no further role in study design, the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication. Francisco Espejo-Porras is a predoctoral fellow supported by the MINECO (FPI Programme). Authors are indebted to Yolanda García-Movellán for administrative assistance.
Disclosure of Potential Conflicts of Interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Eva de Lago and Javier Fernández-Ruiz shared the senior authorship of this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
Representative blots for CB2 receptors and FAAH, always corresponding to tissues from females. Bottom panels are the total protein bands transferred to a PVCF membrane that were used as loading controls. (PPTX 3947 kb)
Figure S2
Behavioral recording in the Water Morris test of TDP-43 transgenic and wild-type male mice at the postsymptomatic (100-110 days after birth) stage. Values are means ± SEM for 6-8 animals per group. Data were assessed by the unpaired Student’s t-test. (PPTX 73 kb)
Figure S3
Immunofluorescence for CB2 receptors (magnification was 40x) showing a complete loss of immunostaining in the spinal cord of CB2 receptor-knockout mice when compared with the signal found in wild-type mice and, in particular, TDP-43 transgenic mice at the postsymptomatic (100-110 days after birth) stage. Immunostainings were repeated in at least 3 animals per group. (PPTX 241 kb)
Rights and permissions
About this article
Cite this article
Espejo-Porras, F., Piscitelli, F., Verde, R. et al. Changes in the endocannabinoid signaling system in CNS structures of TDP-43 transgenic mice: relevance for a neuroprotective therapy in TDP-43-related disorders. J Neuroimmune Pharmacol 10, 233–244 (2015). https://doi.org/10.1007/s11481-015-9602-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-015-9602-4