Abstract
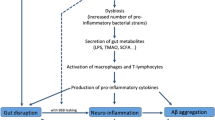
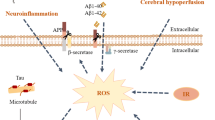
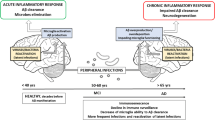
Alzheimer’s disease (AD) is a most common neurodegenerative disorder, which associates with impaired cognition. Gut microbiota can modulate host brain function and behavior via microbiota-gut-brain axis, including cognitive behavior. Germ-free animals, antibiotics, probiotics intervention and diet can induce alterations of gut microbiota and gut physiology and also host cognitive behavior, increasing or decreasing risks of AD. The increased permeability of intestine and blood-brain barrier induced by gut microbiota disturbance will increase the incidence of neurodegeneration disorders. Gut microbial metabolites and their effects on host neurochemical changes may increase or decrease the risk of AD. Pathogenic microbes infection will also increase the risk of AD, and meanwhile, the onset of AD support the “hygiene hypothesis”. All the results suggest that AD may begin in the gut, and is closely related to the imbalance of gut microbiota. Modulation of gut microbiota through personalized diet or beneficial microbiota intervention will probably become a new treatment for AD.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Alonso, R., Pisa, D., Marina, A.I., Morato, E., Rabano, A., and Carrasco, L. (2014a). Fungal infection in patients with Alzheimer’s disease. J Alzheimers Dis 41, 301–311.
Alonso, R., Pisa, D., Rabano, A., and Carrasco, L. (2014b). Alzheimer’s disease and disseminated mycoses. Eur J Clin Microbiol Infect Dis 33, 1125–1132.
Alzheimer’s Association. (2015). 2015 Alzheimer’s disease facts and figures. Alzheimer’s Dementia 11, 332–384.
Apter, A.J. (2003). Early exposure to allergen: is this the cat’s meow, or are we barking up the wrong tree? J Allergy Clin Immunol 111, 938–946.
Arumugam, M., Raes, J., Pelletier, E., Le Paslier, D., Yamada, T., Mende, D.R., Fernandes, G.R., Tap, J., Bruls, T., Batto, J.M., Bertalan, M., Borruel, N., Casellas, F., Fernandez, L., Gautier, L., Hansen, T., Hattori, M., Hayashi, T., Kleerebezem, M., Kurokawa, K., Leclerc, M., Levenez, F., Manichanh, C., Nielsen, H.B., Nielsen, T., Pons, N., Poulain, J., Qin, J.J., Sicheritz-Ponten, T., Tims, S., Torrents, D., Ugarte, E., Zoetendal, E.G., Wang, J., Guarner, F., Pedersen, O., de Vos, W.M., Brunak, S., Dore, J., Weissenbach, J., Ehrlich, S.D., Bork, P., and Consortium, M. (2011). Enterotypes of the human gut microbiome. Nature 473, 174–180.
Aziz, Q., Dore, J., Emmanuel, A., Guarner, F., and Quigley, E.M. (2013). Gut microbiota and gastrointestinal health: current concepts and future directions. Neurogastroenterol Motil 25, 4–15.
Backhed, F., Ley, R.E., Sonnenburg, J.L., Peterson, D.A., and Gordon, J.I. (2005). Host-bacterial mutualism in the human intestine. Science 307, 1915–1920.
Bajaj, J.S., Heuman, D.M., Sanyal, A.J., Hylemon, P.B., Sterling, R.K., Stravitz, R.T., Fuchs, M., Ridlon, J.M., Daita, K., Monteith, P., Noble, N.A., White, M.B., Fisher, A., Sikaroodi, M., Rangwala, H., and Gillevet, P.M. (2013). Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLoS One 8, e60042.
Bajaj, J.S., Ridlon, J.M., Hylemon, P.B., Thacker, L.R., Heuman, D.M., Smith, S., Sikaroodi, M., and Gillevet, P.M. (2012). Linkage of gut microbiome with cognition in hepatic encephalopathy. Am J Physiol-Gastr L 302, G168–G175.
Balin, B.J., Gerard, H.C., Arking, E.J., Appelt, D.M., Branigan, P.J., Abrams, J.T., Whittum-Hudson, J.A., and Hudson, A.P. (1998). Identification and localization of Chlamydia pneumoniae in the Alzheimer’s brain. Med Microbiol Immunol 187, 23–42.
Balin, B.J., and Hudson, A.P. (2014). Etiology and pathogenesis of late-onset Alzheimer’s disease. Curr Allergy Asthma Rep 14, 417.
Ball, M.J. (1982). Limbic predilection in Alzheimer dementia: is reactivated herpes virus involved. Can J Neurol Sci 9, 303–306.
Banack, S.A., Caller, T.A., and Stommel, E.W. (2010). The cyanobacteria derived toxin Beta-N-methylamino-L-alanine and amyotrophic lateral sclerosis. Toxins (Basel) 2, 2837–2850.
Barberger-Gateau, P., Letenneur, L., Deschamps, V., Peres, K., Dartigues, J.F., and Renaud, S. (2002). Fish, meat, and risk of dementia: cohort study. BMJ 325, 932–933.
Barberger-Gateau, P., Raffaitin, C., Letenneur, L., Berr, C., Tzourio, C., Dartigues, J.F., and Alperovitch, A. (2007). Dietary patterns and risk of dementia: the three-city cohort study. Neurology 69, 1921–1930.
Barrett, E., Ross, R.P., O’Toole, P.W., Fitzgerald, G.F., and Stanton, C. (2012). gamma-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol 113, 411–417.
Benjamin, J., Singla, V., Arora, I., Sood, S., and Joshi, Y.K. (2013). Intestinal permeability and complications in liver cirrhosis: a prospective cohort study. Hepatol Res 43, 200–207.
Berstad, A., Arslan, G., and Folvik, G. (2000). Relationship between intestinal permeability and calprotectin concentration in gut lavage fluid. Scand J Gastroenterol 35, 64–69.
Bhattacharjee, S., and Lukiw, W.J. (2013). Alzheimer’s disease and the microbiome. Front Cell Neurosci 7, 153.
Biagi, E., Candela, M., Turroni, S., Garagnani, P., Franceschi, C., and Brigidi, P. (2013). Ageing and gut microbes: perspectives for health maintenance and longevity. Pharmacol Res 69, 11–20.
Biagi, E., Nylund, L., Candela, M., Ostan, R., Bucci, L., Pini, E., Nikkila, J., Monti, D., Satokari, R., Franceschi, C., Brigidi, P., and De Vos, W. (2010). Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One 5, e10667.
Boelen, E., Steinbusch, H.W., van der Ven, A.J., Grauls, G., Bruggeman, C.A., and Stassen, F.R. (2007). Chlamydia pneumoniae infection of brain cells: an in vitro study. Neurobiol Aging 28, 524–532.
Borjabad, A., and Volsky, D.J. (2012). Common transcriptional signatures in brain tissue from patients with HIV-associated neurocognitive disorders, Alzheimer’s disease, and Multiple Sclerosis. J Neuroimmune Pharmacol 7, 914–926.
Borre, Y.E., O’Keeffe, G.W., Clarke, G., Stanton, C., Dinan, T.G., and Cryan, J.F. (2014). Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med 20, 509–518.
Braniste, V., Al-Asmakh, M., Kowal, C., Anuar, F., Abbaspour, A., Toth, M., Korecka, A., Bakocevic, N., Guan, N.L., Kundu, P., Gulyas, B., Halldin, C., Hultenby, K., Nilsson, H., Hebert, H., Volpe, B.T., Diamond, B., and Pettersson, S. (2014). The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med 6, 263ra158.
Bravo, J.A., Julio-Pieper, M., Forsythe, P., Kunze, W., Dinan, T.G., Bienenstock, J., and Cryan, J.F. (2012). Communication between gastrointestinal bacteria and the nervous system. Curr Opin Pharmacol 12, 667–672.
Brenner, S.R. (2013). Blue-green algae or cyanobacteria in the intestinal micro-flora may produce neurotoxins such as Beta-N-methylamino-L-alanine (BMAA) which may be related to development of amyotrophic lateral sclerosis, Alzheimer’s disease and Parkinson-Dementia-Complex in humans and Equine Motor Neuron Disease in horses. Med Hypotheses 80, 103.
Bruce-Keller, A.J., Salbaum, J.M., Luo, M., Blanchard, E.t., Taylor, C.M., Welsh, D.A., and Berthoud, H.R. (2015). Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol Psychiatry 77, 607–615.
Butterfield, D.A., Perluigi, M., and Sultana, R. (2006). Oxidative stress in Alzheimer’s disease brain: new insights from redox proteomics. Eur J Pharmacol 545, 39–50.
Cani, P.D., Bibiloni, R., Knauf, C., Waget, A., Neyrinck, A.M., Delzenne, N.M., and Burcelin, R. (2008). Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57, 1470–1481.
Carlino, D., De Vanna, M., and Tongiorgi, E. (2013). Is altered BDNF biosynthesis a general feature in patients with cognitive dysfunctions? Neuroscientist 19, 345–353.
Castaneda, A.E., Tuulio-Henriksson, A., Aronen, E.T., Marttunen, M., and Kolho, K.L. (2013). Cognitive functioning and depressive symptoms in adolescents with inflammatory bowel disease. World J Gastroenterol 19, 1611–1617.
Chiu, W.C., Tsan, Y.T., Tsai, S.L., Chang, C.J., Wang, J.D., Chen, P.C., and Health Data Analysis in Taiwan Research Group. (2014). Hepatitis C viral infection and the risk of dementia. Eur J Neurol 21, 1068–e1059.
Cho, I., and Blaser, M.J. (2012). The human microbiome: at the interface of health and disease. Nat Rev Genet 13, 260–270.
Cirrito, J.R., Disabato, B.M., Restivo, J.L., Verges, D.K., Goebel, W.D., Sathyan, A., Hayreh, D., D'Angelo, G., Benzinger, T., Yoon, H., Kim, J., Morris, J.C., Mintun, M.A., and Sheline, Y.I. (2011). Serotonin signaling is associated with lower amyloid-beta levels and plaques in transgenic mice and humans. Proc Natl Acad Sci USA 108, 14968–14973.
Claesson, M.J., Cusack, S., O’Sullivan, O., Greene-Diniz, R., de Weerd, H., Flannery, E., Marchesi, J.R., Falush, D., Dinan, T., Fitzgerald, G., Stanton, C., van Sinderen, D., O’Connor, M., Harnedy, N., O’Connor, K., Henry, C., O’Mahony, D., Fitzgerald, A.P., Shanahan, F., Twomey, C., Hill, C., Ross, R.P., and O’Toole, P.W. (2011). Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci USA 108, 4586–4591.
Claesson, M.J., Jeffery, I.B., Conde, S., Power, S.E., O’Connor, E.M., Cusack, S., Harris, H.M.B., Coakley, M., Lakshminarayanan, B., O'Sullivan, O., Fitzgerald, G.F., Deane, J., O’Connor, M., Harnedy, N., O’Connor, K., O'Mahony, D., van Sinderen, D., Wallace, M., Brennan, L., Stanton, C., Marchesi, J.R., Fitzgerald, A.P., Shanahan, F., Hill, C., Ross, R.P., and O’Toole, P.W. (2012). Gut microbiota composition correlates with diet and health in the elderly. Nature 488, 178–184.
Clarke, G., Grenham, S., Scully, P., Fitzgerald, P., Moloney, R.D., Shanahan, F., Dinan, T.G., and Cryan, J.F. (2013). The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry 18, 666–673.
Clemente, J.C., Ursell, L.K., Parfrey, L.W., and Knight, R. (2012). The impact of the gut microbiota on human health: an integrative view. Cell 148, 1258–1270.
Collins, S.M., Surette, M., and Bercik, P. (2012). The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol 10, 735–742.
Cookson, W.O.C.M., and Moffatt, M.F. (1997). Asthma: an epidemic in the absence of infection? Science 275, 41–42.
Cowan, T.E., Palmnas, M.S.A., Yang, J., Bomhof, M.R., Ardell, K.L., Reimer, R.A., Vogel, H.J., and Shearer, J. (2014). Chronic coffee consumption in the diet-induced obese rat: impact on gut microbiota and serum metabolomics. J Nutr Biochem 25, 489–495.
Cox, P.A., Davis, D.A., Mash, D.C., Metcalf, J.S., and Banack, S.A. (2016). Dietary exposure to an environmental toxin triggers neurofibrillary tangles and amyloid deposits in the brain. Proc Biol Sci 283, 20152397.
Crane, P.K., Walker, R., Hubbard, R.A., Li, G., Nathan, D.M., Zheng, H., Haneuse, S., Craft, S., Montine, T.J., Kahn, S.E., McCormick, W., McCurry, S.M., Bowen, J.D., and Larson, E.B. (2013). Glucose levels and risk of dementia. N Engl J Med 369, 540–548.
Crogan, N.L., and Evans, B.C. (2007). Clostridium difficile: an emerging epidemic in nursing homes. Geriatr Nurs 28, 161–164.
Cryan, J.F., and Dinan, T.G. (2012). Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 13, 701–712.
Cryan, J.F., and O’Mahony, S.M. (2011). The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol Motil 23, 187–192.
D’Andrea, M.R. (2005). Add Alzheimer’s disease to the list of autoimmune diseases. Med Hypotheses 64, 458–463.
Davari, S., Talaei, S.A., Alaei, H., and Salami, M. (2013). Probiotics treatment improves diabetes-induced impairment of synaptic activity and cognitive function: behavioral and electrophysiological proofs for microbiome-gut-brain axis. Neuroscience 240, 287–296.
Deane, R., Wu, Z.H., and Zlokovic, B.V. (2004). RAGE (Yin) versus LRP (Yang) balance regulates Alzheimer amyloid beta-peptide clearance through transport across the blood-brain barrier. Stroke 35, 2628–2631.
Dekaban, A.S. (1978). Changes in brain weights during the span of human life: relation of brain weights to body heights and body weights. Ann Neurol 4, 345–356.
Del Chierico, F., Vernocchi, P., Dallapiccola, B., and Putignani, L. (2014). Mediterranean diet and health: food effects on gut microbiota and disease control. Int J of Mol Sci 15, 11678–11699.
Diaz Heijtz, R., Wang, S., Anuar, F., Qian, Y., Bjorkholm, B., Samuelsson, A., Hibberd, M.L., Forssberg, H., and Pettersson, S. (2011). Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA 108, 3047–3052.
Duthie, S.J., Whalley, L.J., Collins, A.R., Leaper, S., Berger, K., and Deary, I.J. (2002). Homocysteine, B vitamin status, and cognitive function in the elderly. Am J Clin Nutr 75, 908–913.
Erny, D., Hrabe de Angelis, A.L., Jaitin, D., Wieghofer, P., Staszewski, O., David, E., Keren-Shaul, H., Mahlakoiv, T., Jakobshagen, K., Buch, T., Schwierzeck, V., Utermohlen, O., Chun, E., Garrett, W.S., McCoy, K.D., Diefenbach, A., Staeheli, P., Stecher, B., Amit, I., and Prinz, M. (2015). Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 18, 965–977.
Eskelinen, M.H., Ngandu, T., Helkala, E.L., Tuomilehto, J., Nissinen, A., Soininen, H., and Kivipelto, M. (2008). Fat intake at midlife and cognitive impairment later in life: a population-based CAIDE study. Int J Geriatr Psychiatry 23, 741–747.
Eskelinen, M.H., Ngandu, T., Tuomilehto, J., Soininen, H., and Kivipelto, M. (2009). Midlife coffee and tea drinking and the risk of late-life dementia: a population-based CAIDE study. J Alzheimers Dis 16, 85–91.
Evenepoel, P., Meijers, B.K.I., Bammens, B.R.M., and Verbeke, K. (2009). Uremic toxins originating from colonic microbial metabolism. Kidney Int 76, S12–S19.
Faria, A.M., de Moraes, S.M., de Freitas, L.H.F., Speciali, E., Soares, T.F., Figueiredo-Neves, S.P., Vitelli-Avelar, D.M., Martins, M.A., Barbosa, K.V.B.D., Soares, E.B., Sathler-Avelar, R., Peruhype-Magalhaes, V., Cardoso, G.M., Comin, F., Teixeira, R., Eloi-Santos, S.M., Queiroz, D.M.M., Correa-Oliveira, R., Bauer, M.E., Teixeira-Carvalho, A., and Martins-Filho, O.A. (2008). Variation rhythms of lymphocyte subsets during healthy aging. Neuroimmunomodulation 15, 365–379.
Finegold, S.M., Dowd, S.E., Gontcharova, V., Liu, C.X., Henley, K.E., Wolcott, R.D., Youn, E., Summanen, P.H., Granpeesheh, D., Dixon, D., Liu, M., Molitoris, D.R., and Green, J.A. (2010). Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe 16, 444–453.
Fontan-Lozano, A., Saez-Cassanelli, J.L., Inda, M.C., Santos-Arteaga, M.D.L., Sierra-Dominguez, S.A., Lopez-Lluch, G., Delgado-Garcia, J.M., and Carrion, A.M. (2007). Caloric restriction increases learning consolidation and facilitates synaptic plasticity through mechanisms dependent on NR2B Subunits of the NMDA receptor. J Neurosci 27, 10185–10195.
Foster, J.A. (2013). Gut feelings: bacteria and the brain. Cerebrum 2013, 9.
Fox, M., Knapp, L.A., Andrews, P.W., and Fincher, C.L. (2013). Hygiene and the world distribution of Alzheimer’s disease: epidemiological evidence for a relationship between microbial environment and age-adjusted disease burden. Evol Med Public Health 2013, 173–186.
Franceschi, C. (2007). Inflammaging as a major characteristic of old people: can it be prevented or cured? Nutr Rev 65, S173–S176.
Gardener, S., Gu, Y., Rainey-Smith, S.R., Keogh, J.B., Clifton, P.M., Mathieson, S.L., Taddei, K., Mondal, A., Ward, V.K., Scarmeas, N., Barnes, M., Ellis, K.A., Head, R., Masters, C.L., Ames, D., Macaulay, S.L., Rowe, C.C., Szoeke, C., Martins, R.N., and Grp, A.R. (2012). Adherence to a Mediterranean diet and Alzheimer’s disease risk in an Australian population. Transl Psychiat 2, e164.
Gareau, M.G., Wine, E., Rodrigues, D.M., Cho, J.H., Whary, M.T., Philpott, D.J., MacQueen, G., and Sherman, P.M. (2011). Bacterial infection causes stress-induced memory dysfunction in mice. Gut 60, 307–317.
Gerard, H.C., Dreses-Werringloer, U., Wildt, K.S., Deka, S., Oszust, C., Balin, B.J., Frey, W.H., Bordayo, E.Z., Whittum-Hudson, J.A., and Hudson, A.P. (2006). Chlamydophila (Chlamydia) pneumoniae in the Alzheimer’s brain. FEMS Immunol Med Microbiol 48, 355–366.
Gomborone, J.E., Dewsnap, P.A., Libby, G.W., and Farthing, M.J.G. (1993). Selective affective biasing in recognition memory in the irritable-bowel-syndrome. Gut 34, 1230–1233.
Gregg, R., Smith, C.M., Clark, F.J., Dunnion, D., Khan, N., Chakraverty, R., Nayak, L., and Moss, P.A. (2005). The number of human peripheral blood CD4+ CD25(high) regulatory T cells increases with age. Clin Exp Immunol 140, 540–546.
Gu, Y.A., Nieves, J.W., Stern, Y., Luchsinger, J.A., and Scarmeas, N. (2010). Food combination and Alzheimer disease risk a protective diet. Arch Neurol 67, 699–706.
Guigoz, Y., Dore, J., and Schiffrina, E.J. (2008). The inflammatory status of old age can be nurtured from the intestinal environment. Curr Opin Clin Nutr Metab Care 11, 13–20.
Heintz, C., and Mair, W. (2014). You are what you host: microbiome modulation of the aging process. Cell 156, 408–411.
Hendrie, H.C., Osuntokun, B.O., Hall, K.S., Ogunniyi, A.O., Hui, S.L., Unverzagt, F.W., Gureje, O., Rodenberg, C.A., Baiyewu, O., and Musick, B.S. (1995). Prevalence of Alzheimer’s disease and dementia in two communities: Nigerian Africans and African Americans. Am J Psychiatry 152, 1485–1492.
Hill, J.M., and Lukiw, W.J. (2015). Microbial-generated amyloids and Alzheimer’s disease (AD). Front Aging Neurosci 7, 9.
Holmqvist, S., Chutna, O., Bousset, L., Aldrin-Kirk, P., Li, W., Bjorklund, T., Wang, Z.Y., Roybon, L., Melki, R., and Li, J.Y. (2014). Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol 128, 805–820.
Hooper, L.V., and Gordon, J.I. (2001). Commensal host-bacterial relationships in the gut. Science 292, 1115–1118.
Huang, W.S., Yang, T.Y., Shen, W.C., Lin, C.L., Lin, M.C., and Kao, C.H. (2014). Association between Helicobacter pylori infection and dementia. J Clin Neurosci 21, 1355–1358.
Hughes, T.F., Andel, R., Small, B.J., Borenstein, A.R., Mortimer, J.A., Wolk, A., Johansson, B., Fratiglioni, L., Pedersen, N.L., and Gatz, M. (2010). Midlife fruit and vegetable consumption and risk of dementia in later life in Swedish twins. Am J Geriatr Psychiatry 18, 413–420.
Human Microbiome Project Consortium. (2012). A framework for human microbiome research. Nature 486, 215–221.
Itzhaki, R.F., and Wozniak, M.A. (2008). Herpes simplex virus type 1 in Alzheimer’s disease: the enemy within. J Alzheimers Dis 13, 393–405.
Jaeger, L.B., Dohgu, S., Sultana, R., Lynch, J.L., Owen, J.B., Erickson, M.A., Shah, G.N., Price, T.O., Fleegal-Demotta, M.A., Butterfiled, D.A., and Banks, W.A. (2009). Lipopolysaccharide alters the blood-brain barrier transport of amyloid beta protein: a mechanism for inflammation in the progression of Alzheimer’s disease. Brain Behav Immun 23, 507–517.
Jakobsson, H.E., Rodriguez-Pineiro, A.M., Schutte, A., Ermund, A., Boysen, P., Bemark, M., Sommer, F., Backhed, F., Hansson, G.C., and Johansson, M.E.V. (2015). The composition of the gut microbiota shapes the colon mucus barrier. Embo Rep 16, 164–177.
Jaquet, M., Rochat, I., Moulin, J., Cavin, C., and Bibiloni, R. (2009). Impact of coffee consumption on the gut microbiota: a human volunteer study. Int J Food Microbiol 130, 117–121.
Jarvis, D., Chinn, S., Luczynska, C., and Burney, P. (1997). The association of family size with atopy and atopic disease. Clin Exp Allergy 27, 240–245.
Kahn, M.S., Kranjac, D., Alonzo, C.A., Haase, J.F., Cedillos, R.O., McLinden, K.A., Boehm, G.W., and Chumley, M.J. (2012). Prolonged elevation in hippocampal A beta and cognitive deficits following repeated endotoxin exposure in the mouse. Behav Brain Res 229, 176–184.
Katan, M., Moon, Y.P., Paik, M.C., Sacco, R.L., Wright, C.B., and Elkind, M.S.V. (2013). Infectious burden and cognitive function The Northern Manhattan Study. Neurology 80, 1209–1215.
Kelly, J.R., Kennedy, P.J., Cryan, J.F., Dinan, T.G., Clarke, G., and Hyland, N. (2015). Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci 9, 392.
Kimball, B.A., Wilson, D.A., and Wesson, D.W. (2016). Alterations of the volatile metabolome in mouse models of Alzheimer’s disease. Sci Rep 6, 19495.
Knight, E.M., Martins, I.V.A., Gumusgoz, S., Allan, S.M., and Lawrence, C.B. (2014). High-fat diet-induced memory impairment in triple-transgenic Alzheimer’s disease (3xTgAD) mice is independent of changes in amyloid and tau pathology. Neurobiol Aging 35, 1821–1832.
Kountouras, J., Boziki, M., Zavos, C., Gavalas, E., Giartza-Taxidou, E., Venizelos, I., Deretzi, G., Grigoriadis, N., Tsiaousi, E., and Vardaka, E. (2012). A potential impact of chronic Helicobacter pylori infection on Alzheimer’s disease pathobiology and course. Neurobiol Aging 33, e3–4.
Kountouras, J., Gavalas, E., Zavos, C., Stergiopoulos, C., Chatzopoulos, D., Kapetanakis, N., and Gisakis, D. (2007). Alzheimer’s disease and Helicobacter pylori infection: defective immune regulation and apoptosis as proposed common links. Med Hypotheses 68, 378–388.
Laitinen, M.H., Ngandu, T., Rovio, S., Helkala, E.L., Uusitalo, U., Viitanen, M., Nissinen, A., Tuomilehto, J., Soininen, H., and Kivipelto, M. (2006). Fat intake at midlife and risk of dementia and Alzheimer’s disease: a population-based study. Dement Geriatr Cogn Disord 22, 99–107.
Lakhan, S.E., Caro, M., and Hadzimichalis, N. (2013). NMDA receptor activity in neuropsychiatric disorders. Front Psychiatry 4, 52.
Lambert, J.C., Ibrahim-Verbaas, C.A., Harold, D., Naj, A.C., Sims, R., Bellenguez, C., DeStafano, A.L., Bis, J.C., Beecham, G.W., Grenier-Boley, B., Russo, G., Thorton-Wells, T.A., Jones, N., Smith, A.V., Chouraki, V., Thomas, C., Ikram, M.A., Zelenika, D., Vardarajan, B.N., Kamatani, Y., Lin, C.F., Gerrish, A., Schmidt, H., Kunkle, B., Dunstan, M.L., Ruiz, A., Bihoreau, M.T., Choi, S.H., Reitz, C., Pasquier, F., Cruchaga, C., Craig, D., Amin, N., Berr, C., Lopez, O.L., De Jager, P.L., Deramecourt, V., Johnston, J.A., Evans, D., Lovestone, S., Letenneur, L., Moron, F.J., Rubinsztein, D.C., Eiriksdottir, G., Sleegers, K., Goate, A.M., Fievet, N., Huentelman, M.W., Gill, M., Brown, K., Kamboh, M.I., Keller, L., Barberger-Gateau, P., McGuiness, B., Larson, E.B., Green, R., Myers, A.J., Dufouil, C., Todd, S., Wallon, D., Love, S., Rogaeva, E., Gallacher, J., St George-Hyslop, P., Clarimon, J., Lleo, A., Bayer, A., Tsuang, D.W., Yu, L., Tsolaki, M., Bossu, P., Spalletta, G., Proitsi, P., Collinge, J., Sorbi, S., Sanchez-Garcia, F., Fox, N.C., Hardy, J., Deniz Naranjo, M.C., Bosco, P., Clarke, R., Brayne, C., Galimberti, D., Mancuso, M., Matthews, F., European Alzheimer’s Disease Initiative, Genetic and Environmental Risk in Alzheimer’s Disease, Alzheimer’s Disease Genetic Consortium, Cohorts for Heart and Aging Research in Genomic Epidemiology, Moebus, S., Mecocci, P., Del Zompo, M., Maier, W., Hampel, H., Pilotto, A., Bullido, M., Panza, F., Caffarra, P., Nacmias, B., Gilbert, J.R., Mayhaus, M., Lannefelt, L., Hakonarson, H., Pichler, S., Carrasquillo, M.M., Ingelsson, M., Beekly, D., Alvarez, V., Zou, F., Valladares, O., Younkin, S.G., Coto, E., Hamilton-Nelson, K.L., Gu, W., Razquin, C., Pastor, P., Mateo, I., Owen, M.J., Faber, K.M., Jonsson, P.V., Combarros, O., O'Donovan, M.C., Cantwell, L.B., Soininen, H., Blacker, D., Mead, S., Mosley, T.H., Jr., Bennett, D.A., Harris, T.B., Fratiglioni, L., Holmes, C., de Bruijn, R.F., Passmore, P., Montine, T.J., Bettens, K., Rotter, J.I., Brice, A., Morgan, K., Foroud, T.M., Kukull, W.A., Hannequin, D., Powell, J.F., Nalls, M.A., Ritchie, K., Lunetta, K.L., Kauwe, J.S., Boerwinkle, E., Riemenschneider, M., Boada, M., Hiltuenen, M., Martin, E.R., Schmidt, R., Rujescu, D., Wang, L.S., Dartigues, J.F., Mayeux, R., Tzourio, C., Hofman, A., Nothen, M.M., Graff, C., Psaty, B.M., Jones, L., Haines, J.L., Holmans, P.A., Lathrop, M., Pericak-Vance, M.A., Launer, L.J., Farrer, L.A., van Duijn, C.M., Van Broeckhoven, C., Moskvina, V., Seshadri, S., Williams, J., Schellenberg, G.D., and Amouyel, P. (2013). Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet 45, 1452–1458.
Lanctot, K.L., Herrmann, N., Mazzotta, P., Khan, L.R., and Ingber, N. (2004). GABAergic function in Alzheimer’s disease: evidence for dysfunction and potential as a therapeutic target for the treatment of behavioural and psychological symptoms of dementia. Can J Psychiat 49, 439–453.
LaRue, B., Hogg, E., Sagare, A., Jovanovic, S., Maness, L., Maurer, C., Deane, R., and Zlokovic, B.V. (2004). Method for measurement of the blood-brain barrier permeability in the perfused mouse brain: application to amyloid-beta peptide in wild type and Alzheimer’s Tg2576 mice. J Neurosci Methods 138, 233–242.
Leblhuber, F., Geisler, S., Steiner, K., Fuchs, D., and Schutz, B. (2015). Elevated fecal calprotectin in patients with Alzheimer’s dementia indicates leaky gut. J Neural Transm 122, 1319–1322.
Lee, J.W., Lee, Y.K., Yuk, D.Y., Choi, D.Y., Ban, S.B., Oh, K.W., and Hong, J.T. (2008). Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J Neuroinflammation 5, 37.
Ley, R.E. (2015). The gene-microbe link. Nature 518, S7.
Ley, R.E., Turnbaugh, P.J., Klein, S., and Gordon, J.I. (2006). Microbial ecology: human gut microbes associated with obesity. Nature 444, 1022–1023.
Li, F., and Tsien, J.Z. (2009). Memory and the NMDA Receptors. N Engl J Med 361, 302–303.
Liang, S., Wang, T., Hu, X., Luo, J., Li, W., Wu, X., Duan, Y., and Jin, F. (2015). Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience 310, 561–577.
Little, C.S., Hammond, C.J., MacIntyre, A., Balin, B.J., and Appelt, D.M. (2004). Chlamydia pneumoniae induces Alzheimer-like amyloid plaques in brains of BALB/c mice. Neurobiol Aging 25, 419–429.
Liu, T.Y., Hougen, H., Vollmer, A.C., and Hiebert, S.M. (2012). Gut bacteria profiles of Mus musculus at the phylum and family levels are influenced by saturation of dietary fatty acids. Anaerobe 18, 331–337.
Llibre Rodriguez, J.J., Ferri, C.P., Acosta, D., Guerra, M., Huang, Y., Jacob, K.S., Krishnamoorthy, E.S., Salas, A., Sosa, A.L., Acosta, I., Dewey, M.E., Gaona, C., Jotheeswaran, A.T., Li, S., Rodriguez, D., Rodriguez, G., Kumar, P.S., Valhuerdi, A., Prince, M., and Dementia Research, G. (2008). Prevalence of dementia in Latin America, India, and China: a population-based cross-sectional survey. Lancet 372, 464–474.
Lukiw, W.J., Cui, J.G., Yuan, L.Y., Bhattacharjee, P.S., Corkern, M., Clement, C., Kammerman, E.M., Ball, M.J., Zhao, Y., Sullivan, P.M., and Hill, J.M. (2010). Acyclovir or Abeta42 peptides attenuate HSV-1-induced miRNA-146a levels in human primary brain cells. Neuroreport 21, 922–927.
Luo, J., Wang, T., Liang, S., Hu, X., Li, W., and Jin, F. (2014). Ingestion of Lactobacillus strain reduces anxiety and improves cognitive function in the hyperammonemia rat. Sci China Life Sci 57, 327–335.
Lurain, N.S., Hanson, B.A., Martinson, J., Leurgans, S.E., Landay, A.L., Bennett, D.A., and Schneider, J.A. (2013). Virological and immunological characteristics of human cytomegalovirus infection associated with Alzheimer disease. J Infect Dis 208, 564–572.
Lynch, N.R., Hagel, I., Perez, M., Di Prisco, M.C., Lopez, R., and Alvarez, N. (1993). Effect of anthelmintic treatment on the allergic reactivity of children in a tropical slum. J Allergy Clin Immunol 92, 404–411.
Mancuso, R., Baglio, F., Cabinio, M., Calabrese, E., Hernis, A., Nemni, R., and Clerici, M. (2014). Titers of herpes simplex virus type 1 antibodies positively correlate with grey matter volumes in Alzheimer’s disease. J Alzheimers Dis 38, 741–745.
Marlow, G., Ellett, S., Ferguson, I.R., Zhu, S.T., Karunasinghe, N., Jesuthasan, A.C., Han, D.Y., Fraser, A.G., and Ferguson, L.R. (2013). Transcriptomics to study the effect of a Mediterranean-inspired diet on inflammation in Crohn’s disease patients. Hum Genomics 7, 24.
Martin, B., Mattson, M.P., and Maudsley, S. (2006). Caloric restriction and intermittent fasting: two potential diets for successful brain aging. Ageing Res Rev 5, 332–353.
Masaki, K.H., Losonczy, K.G., Izmirlian, G., Foley, D.J., Ross, G.W., Petrovitch, H., Havlik, R., and White, L.R. (2000). Association of vitamin E and C supplement use with cognitive function and dementia in elderly men. Neurology 54, 1265–1272.
Matricardi, P.M., Franzinelli, F., Franco, A., Caprio, G., Murru, F., Cioffi, D., Ferrigno, L., Palermo, A., Ciccarelli, N., and Rosmini, F. (1998). Sibship size, birth order, and atopy in 11,371 Italian young men. J Allergy Clin Immunol 101, 439–444.
Matsumoto, M., Kibe, R., Ooga, T., Aiba, Y., Sawaki, E., Koga, Y., and Benno, Y. (2013). Cerebral low-molecular metabolites influenced by intestinal microbiota: a pilot study. Front Syst Neurosci 7, 9.
Messaoudi, M., Lalonde, R., Violle, N., Javelot, H., Desor, D., Nejdi, A., Bisson, J.F., Rougeot, C., Pichelin, M., Cazaubiel, M., and Cazaubiel, J.M. (2011). Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr 105, 755–764.
Miklossy, J. (2011). Emerging roles of pathogens in Alzheimer disease. Expert Rev Mol Med 13, e30.
Minter, M.R., Zhang, C., Leone, V., Ringus, D.L., Zhang, X.Q., Oyler-Castrillo, P., Musch, M.W., Liao, F., Ward, J.F., Holtzman, D.M., Chang, E.B., Tanzi, R.E., and Sisodia, S.S. (2016). Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amylodosis in a murine model of Alzheimer’s disease. Sci Rep 6, 30028.
Moco, S., Martin, F.P.J., and Rezzi, S. (2012). Metabolomics view on gut microbiome modulation by polyphenol-rich foods. J Proteome Res 11, 4781–4790.
Morris, M.C., Evans, D.A., Bienias, J.L., Tangney, C.C., Bennett, D.A., Aggarwal, N., Wilson, R.S., and Scherr, P.A. (2002). Dietary intake of antioxidant nutrients and the risk of incident Alzheimer disease in a biracial community study. JAMA 287, 3230–3237.
Morris, M.C., Evans, D.A., Bienias, J.L., Tangney, C.C., Bennett, D.A., Wilson, R.S., Aggarwal, N., and Schneider, J. (2003). Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol 60, 940–946.
Mulligan, V.K., and Chakrabartty, A. (2013). Protein misfolding in the late-onset neurodegenerative diseases: common themes and the unique case of amyotrophic lateral sclerosis. Proteins 81, 1285–1303.
Murphy, M.C., and Fox, E.A. (2010). Mice deficient in brain-derived neurotrophic factor have altered development of gastric vagal sensory innervation. J Comp Neurol 518, 2934–2951.
Naseribafrouei, A., Hestad, K., Avershina, E., Sekelja, M., Linlokken, A., Wilson, R., and Rudi, K. (2014). Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil 26, 1155–1162.
Neufeld, K.M., Kang, N., Bienenstock, J., and Foster, J.A. (2011). Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil 23, 255–264, e119.
O'Toole, P.W., and Claesson, M.J. (2010). Gut microbiota: changes throughout the lifespan from infancy to elderly. Int Dairy J 20, 281–291.
Ohsawa, K., Uchida, N., Ohki, K., Nakamura, Y., and Yokogoshi, H. (2015). Lactobacillus helveticus-fermented milk improves learning and memory in mice. Nutr Neurosci 18, 232–240.
Patel, N.V., Gordon, M.N., Connor, K.E., Good, R.A., Engelman, R.W., Mason, J., Morgan, D.G., Morgan, T.E., and Finch, C.E. (2005). Caloric restriction attenuates A beta-deposition in Alzheimer transgenic models. Neurobiol Aging 26, 995–1000.
Pautas, E., Cherin, P., De Jaeger, C., and Godeau, P. (1999). Vitamin B12 deficiency in the elderly. Presse Med 28, 1767–1770.
Pellicano, M., Larbi, A., Goldeck, D., Colonna-Romano, G., Buffa, S., Bulati, M., Rubino, G., Iemolo, F., Candore, G., Caruso, C., Derhovanessian, E., and Pawelec, G. (2012). Immune profiling of Alzheimer patients. J Neuroimmunol 242, 52–59.
Pisa, D., Alonso, R., Rabano, A., Rodal, I., and Carrasco, L. (2015). Different brain regions are infected with fungi in Alzheimer’s disease. Sci Rep 5, 15015.
Poole, S., Singhrao, S.K., Kesavalu, L., Curtis, M.A., and Crean, S. (2013). Determining the presence of periodontopathic virulence factors in short-term postmortem Alzheimer’s disease brain tissue. J Alzheimers Dis 36, 665–677.
Prandota, J. (2014). Possible link between toxoplasma gondii and the anosmia associated with neurodegenerative diseases. Am J Alzheimers Dis 29, 205–214.
Prasad, S., Dhiman, R.K., Duseja, A., Chawla, Y.K., Sharma, A., and Agarwal, R. (2007). Lactulose improves cognitive functions and health-related quality of life in patients with cirrhosis who have minimal hepatic encephalopathy. Hepatology 45, 549–559.
Prescott, S.L. (2008). Promoting tolerance in early life: pathways and pitfalls. Curr Allergy Clin Im 21, 64–69.
Prince, M., Bryce, R., Albanese, E., Wimo, A., Ribeiro, W., and Ferri, C.P. (2013). The global prevalence of dementia: a systematic review and metaanalysis. Alzheimer’s Dementia 9, 63–75.
Qin, J., Li, R., Raes, J., Arumugam, M., Burgdorf, K.S., Manichanh, C., Nielsen, T., Pons, N., Levenez, F., Yamada, T., Mende, D.R., Li, J., Xu, J., Li, S., Li, D., Cao, J., Wang, B., Liang, H., Zheng, H., Xie, Y., Tap, J., Lepage, P., Bertalan, M., Batto, J.M., Hansen, T., Le Paslier, D., Linneberg, A., Nielsen, H.B., Pelletier, E., Renault, P., Sicheritz-Ponten, T., Turner, K., Zhu, H., Yu, C., Li, S., Jian, M., Zhou, Y., Li, Y., Zhang, X., Li, S., Qin, N., Yang, H., Wang, J., Brunak, S., Dore, J., Guarner, F., Kristiansen, K., Pedersen, O., Parkhill, J., Weissenbach, J., Meta, H.I.T.C., Bork, P., Ehrlich, S.D., and Wang, J. (2010). A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65.
Qin, J., Li, Y., Cai, Z., Li, S., Zhu, J., Zhang, F., Liang, S., Zhang, W., Guan, Y., Shen, D., Peng, Y., Zhang, D., Jie, Z., Wu, W., Qin, Y., Xue, W., Li, J., Han, L., Lu, D., Wu, P., Dai, Y., Sun, X., Li, Z., Tang, A., Zhong, S., Li, X., Chen, W., Xu, R., Wang, M., Feng, Q., Gong, M., Yu, J., Zhang, Y., Zhang, M., Hansen, T., Sanchez, G., Raes, J., Falony, G., Okuda, S., Almeida, M., LeChatelier, E., Renault, P., Pons, N., Batto, J.M., Zhang, Z., Chen, H., Yang, R., Zheng, W., Li, S., Yang, H., Wang, J., Ehrlich, S.D., Nielsen, R., Pedersen, O., Kristiansen, K., and Wang, J. (2012). A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490, 55–60.
Qin, N., Yang, F.L., Li, A., Prifti, E., Chen, Y.F., Shao, L., Guo, J., Le Chatelier, E., Yao, J., Wu, L.J., Zhou, J.W., Ni, S.J., Liu, L., Pons, N., Batto, J.M., Kennedy, S.P., Leonard, P., Yuan, C.H., Ding, W.C., Chen, Y.T., Hu, X.J., Zheng, B.W., Qian, G.R., Xu, W., Ehrlich, S.D., Zheng, S.S., and Li, L.J. (2014). Alterations of the human gut microbiome in liver cirrhosis. Nature 513, 59–64.
Quadri, P., Fragiacomo, C., Pezzati, R., Zanda, E., Forloni, G., Tettamanti, M., and Lucca, U. (2004). Homocysteine, folate, and vitamin B-12 in mild cognitive impairment, Alzheimer disease, and vascular dementia. Am J Clin Nutr 80, 114–122.
Refolo, L.M., Malester, B., LaFrancois, J., Bryant-Thomas, T., Wang, R., Tint, G.S., Sambamurti, K., Duff, K., and Pappolla, M.A. (2001). A high fat, high cholesterol diet accelerates beta-amyloid accumulation in the CNS of a transgenic mouse model of Alzheimer’s disease. Alzheimer’s Disease, 433–447.
Reitz, C., Brayne, C., and Mayeux, R. (2011). Epidemiology of Alzheimer disease. Nat Rev Neurol 7, 137–152.
Romagnani, S. (2004). The increased prevalence of allergy and the hygiene hypothesis: missing immune deviation, reduced immune suppression, or both? Immunology 112, 352–363.
Rook, G.A. (2007). The hygiene hypothesis and the increasing prevalence of chronic inflammatory disorders. Trans R Soc Trop Med Hyg 101, 1072–1074.
Rook, G.A. (2012). Hygiene hypothesis and autoimmune diseases. Clin Rev Allergy Immunol 42, 5–15.
Rook, G.A., and Lowry, C.A. (2008). The hygiene hypothesis and psychiatric disorders. Trends Immunol 29, 150–158.
Rook, G.A.W. (2009). Introduction: the changing microbial environment, Darwinian medicine and the hygiene hypothesis. The Hygiene Hypothesis and Darwinian Medicine. (Basel: Springer) pp. 1–27.
Roubaud-Baudron, C., Krolak-Salmon, P., Quadrio, I., Megraud, F., and Salles, N. (2012). Impact of chronic Helicobacter pylori infection on Alzheimer’s disease: preliminary results. Neurobiol Aging 33, e11–e19.
Sampson, T.R., and Mazmanian, S.K. (2015). Control of brain development, function, and behavior by the microbiome. Cell Host Microbe 17, 565–576.
Saresella, M., Calabrese, E., Marventano, I., Piancone, F., Gatti, A., Calvo, M.G., Nemni, R., and Clerici, M. (2010). PD1 negative and PD1 positive CD4+T regulatory cells in mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis 21, 927–938.
Savignac, H.M., Tramullas, M., Kiely, B., Dinan, T.G., and Cryan, J.F. (2015). Bifidobacteria modulate cognitive processes in an anxious mouse strain. Behav Brain Res 287, 59–72.
Scarmeas, N., Stern, Y., Tang, M.X., Mayeux, R., and Luchsinger, J.A. (2006). Mediterranean diet and risk for Alzheimer’s disease. Ann Neurol 59, 912–921.
Scheperjans, F., Aho, V., Pereira, P.A.B., Koskinen, K., Paulin, L., Pekkonen, E., Haapaniemi, E., Kaakkola, S., Eerola-Rautio, J., Pohja, M., Kinnunen, E., Murros, K., and Auvinen, P. (2015). Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord 30, 350–358.
Schuijt, T.J., Lankelma, J.M., Scicluna, B.P., de Sousa, E.M.F., Roelofs, J.J., de Boer, J.D., Hoogendijk, A.J., de Beer, R., de Vos, A., Belzer, C., de Vos, W.M., van der Poll, T., and Wiersinga, W.J. (2016). The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut 65, 575–583.
Schwarz, M.J., Chiang, S., Muller, N., and Ackenheil, M. (2001). T-helper-1 and T-helper-2 responses in psychiatric disorders. Brain Behav Immun 15, 340–370.
Sekirov, I., Tam, N.M., Jogova, M., Robertson, M.L., Li, Y., Lupp, C., and Finlay, B.B. (2008). Antibiotic-induced perturbations of the intestinal microbiota alter host susceptibility to enteric infection. Infect Immun 76, 4726–4736.
Sidhu, S.S., Goyal, O., Mishra, B.P., Sood, A., Chhina, R.S., and Soni, R.K. (2011). Rifaximin improves psychometric performance and health-related quality of life in patients with minimal hepatic encephalopathy (the RIME trial). Am J Gastroenterol 106, 307–316.
Solakivi, T., Kaukinen, K., Kunnas, T., Lehtimaki, T., Maki, M., and Nikkari, S.T. (2011). Serum fatty acid profile in subjects with irritable bowel syndrome. Scand J Gastroenterol 46, 299–303.
Solas, M., Puerta, E., and Ramirez, M.J. (2015). Treatment options in Alzheimer’s disease: the GABA story. Curr Pharm Des 21, 4960–4971.
Solfrizzi, V., Colacicco, A.M., D'Introno, A., Capurso, C., Torres, F., Rizzo, C., Capurso, A., and Panza, F. (2006). Dietary intake of unsaturated fatty acids and age-related cognitive decline: a 8.5-year follow-up of the Italian Longitudinal Study on Aging. Neurobiol Aging 27, 1694–1704.
Sowell, E.R., Peterson, B.S., Thompson, P.M., Welcome, S.E., Henkenius, A.L., and Toga, A.W. (2003). Mapping cortical change across the human life span. Nat Neurosci 6, 309–315.
Strachan, D.P. (1989). Hay fever, hygiene, and household size. BMJ 299, 1259–1260.
Strandberg, T.E., Pitkala, K.H., Linnavuori, K.H., and Tilvis, R.S. (2003). Impact of viral and bacterial burden on cognitive impairment in elderly persons with cardiovascular diseases. Stroke 34, 2126–2131.
Sudo, N., Chida, Y., Aiba, Y., Sonoda, J., Oyama, N., Yu, X.N., Kubo, C., and Koga, Y. (2004). Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol-London 558, 263–275.
Tilg, H., and Moschen, A.R. (2014). Microbiota and diabetes: an evolving relationship. Gut 63, 1513–1521.
Tillisch, K., Labus, J., Kilpatrick, L., Jiang, Z., Stains, J., Ebrat, B., Guyonnet, D., Legrain-Raspaud, S., Trotin, B., Naliboff, B., and Mayer, E.A. (2013). Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 144, 1394–1401.
Tran, L., and Greenwood-Van Meerveld, B. (2013). Age-associated remodeling of the intestinal epithelial barrier. J Gerontol A Biol Sci Med Sci 68, 1045–1056.
Tully, A.M., Roche, H.M., Doyle, R., Fallon, C., Bruce, I., Lawlor, B., Coakley, D., and Gibney, M.J. (2003). Low serum cholesteryl ester-docosahexaenoic acid levels in Alzheimer’s disease: a case-control study. Br J Nutr 89, 483–489.
Turner, J.R. (2009). Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 9, 799–809.
Vanhanen, M., Kuusisto, J., Koivisto, K., Mykkanen, L., Helkala, E.L., Hanninen, T., Riekkinen, P., Sr., Soininen, H., and Laakso, M. (1999). Type-2 diabetes and cognitive function in a non-demented population. Acta Neurol Scand 100, 97–101.
Venter, J.C., Adams, M.D., Myers, E.W., Li, P.W., Mural, R.J., Sutton, G.G., Smith, H.O., Yandell, M., Evans, C.A., Holt, R.A., Gocayne, J.D., Amanatides, P., Ballew, R.M., Huson, D.H., Wortman, J.R., Zhang, Q., Kodira, C.D., Zheng, X.H., Chen, L., Skupski, M., Subramanian, G., Thomas, P.D., Zhang, J., Gabor Miklos, G.L., Nelson, C., Broder, S., Clark, A.G., Nadeau, J., McKusick, V.A., Zinder, N., Levine, A.J., Roberts, R.J., Simon, M., Slayman, C., Hunkapiller, M., Bolanos, R., Delcher, A., Dew, I., Fasulo, D., Flanigan, M., Florea, L., Halpern, A., Hannenhalli, S., Kravitz, S., Levy, S., Mobarry, C., Reinert, K., Remington, K., Abu-Threideh, J., Beasley, E., Biddick, K., Bonazzi, V., Brandon, R., Cargill, M., Chandramouliswaran, I., Charlab, R., Chaturvedi, K., Deng, Z., Di Francesco, V., Dunn, P., Eilbeck, K., Evangelista, C., Gabrielian, A.E., Gan, W., Ge, W., Gong, F., Gu, Z., Guan, P., Heiman, T.J., Higgins, M.E., Ji, R.R., Ke, Z., Ketchum, K.A., Lai, Z., Lei, Y., Li, Z., Li, J., Liang, Y., Lin, X., Lu, F., Merkulov, G.V., Milshina, N., Moore, H.M., Naik, A.K., Narayan, V.A., Neelam, B., Nusskern, D., Rusch, D.B., Salzberg, S., Shao, W., Shue, B., Sun, J., Wang, Z., Wang, A., Wang, X., Wang, J., Wei, M., Wides, R., Xiao, C., Yan, C., Yao, A., Ye, J., Zhan, M., Zhang, W., Zhang, H., Zhao, Q., Zheng, L., Zhong, F., Zhong, W., Zhu, S., Zhao, S., Gilbert, D., Baumhueter, S., Spier, G., Carter, C., Cravchik, A., Woodage, T., Ali, F., An, H., Awe, A., Baldwin, D., Baden, H., Barnstead, M., Barrow, I., Beeson, K., Busam, D., Carver, A., Center, A., Cheng, M.L., Curry, L., Danaher, S., Davenport, L., Desilets, R., Dietz, S., Dodson, K., Doup, L., Ferriera, S., Garg, N., Gluecksmann, A., Hart, B., Haynes, J., Haynes, C., Heiner, C., Hladun, S., Hostin, D., Houck, J., Howland, T., Ibegwam, C., Johnson, J., Kalush, F., Kline, L., Koduru, S., Love, A., Mann, F., May, D., McCawley, S., McIntosh, T., McMullen, I., Moy, M., Moy, L., Murphy, B., Nelson, K., Pfannkoch, C., Pratts, E., Puri, V., Qureshi, H., Reardon, M., Rodriguez, R., Rogers, Y.H., Romblad, D., Ruhfel, B., Scott, R., Sitter, C., Smallwood, M., Stewart, E., Strong, R., Suh, E., Thomas, R., Tint, N.N., Tse, S., Vech, C., Wang, G., Wetter, J., Williams, S., Williams, M., Windsor, S., Winn-Deen, E., Wolfe, K., Zaveri, J., Zaveri, K., Abril, J.F., Guigo, R., Campbell, M.J., Sjolander, K.V., Karlak, B., Kejariwal, A., Mi, H., Lazareva, B., Hatton, T., Narechania, A., Diemer, K., Muruganujan, A., Guo, N., Sato, S., Bafna, V., Istrail, S., Lippert, R., Schwartz, R., Walenz, B., Yooseph, S., Allen, D., Basu, A., Baxendale, J., Blick, L., Caminha, M., Carnes-Stine, J., Caulk, P., Chiang, Y.H., Coyne, M., Dahlke, C., Mays, A., Dombroski, M., Donnelly, M., Ely, D., Esparham, S., Fosler, C., Gire, H., Glanowski, S., Glasser, K., Glodek, A., Gorokhov, M., Graham, K., Gropman, B., Harris, M., Heil, J., Henderson, S., Hoover, J., Jennings, D., Jordan, C., Jordan, J., Kasha, J., Kagan, L., Kraft, C., Levitsky, A., Lewis, M., Liu, X., Lopez, J., Ma, D., Majoros, W., McDaniel, J., Murphy, S., Newman, M., Nguyen, T., Nguyen, N., Nodell, M., Pan, S., Peck, J., Peterson, M., Rowe, W., Sanders, R., Scott, J., Simpson, M., Smith, T., Sprague, A., Stockwell, T., Turner, R., Venter, E., Wang, M., Wen, M., Wu, D., Wu, M., Xia, A., Zandieh, A., and Zhu, X. (2001). The sequence of the human genome. Science 291, 1304–1351.
von Mutius, E., Martinez, F.D., Fritzsch, C., Nicolai, T., Reitmeir, P., and Thiemann, H.H. (1994). Skin test reactivity and number of siblings. BMJ 308, 692–695.
von Mutius, E., and Vercelli, D. (2010). Farm living: effects on childhood asthma and allergy. Nat Rev Immunol 10, 861–868.
Vonmutius, E., Fritzsch, C., Weiland, S.K., Roll, G., and Magnussen, H. (1992). Prevalence of asthma and allergic disorders among children in United Germany: a descriptive comparison. Br Med J 305, 1395–1399.
Wang, T., Hu, X., Liang, S., Li, W., Wu, X., Wang, L., and Jin, F. (2015). Lactobacillus fermentum NS9 restores the antibiotic induced physiological and psychological abnormalities in rats. Benef Microbes 6, 707–717.
Welling, M.M., Nabuurs, R.J., and van der Weerd, L. (2015). Potential role of antimicrobial peptides in the early onset of Alzheimer's disease. Alzheimer’s Dementia 11, 51–57.
Widera, M., Klein, A.N., Cinar, Y., Funke, S.A., Willbold, D., and Schaal, H. (2014). The D-amino acid peptide D3 reduces amyloid fibril boosted HIV-1 infectivity. AIDS Res Ther 11, 1.
Willett, W.C., Sacks, F., Trichopoulou, A., Drescher, G., Ferroluzzi, A., Helsing, E., and Trichopoulos, D. (1995). Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr 61, 1402s–1406s.
Witte, A.V., Fobker, M., Gellner, R., Knecht, S., and Floel, A. (2009). Caloric restriction improves memory in elderly humans. Proc Natl Acad Sci USA 106, 1255–1260.
Wozniak, M.A., Frost, A.L., and Itzhaki, R.F. (2009a). Alzheimer’s disease-specific Tau phosphorylation is induced by herpes simplex virus type 1. J Alzheimers Dis 16, 341–350.
Wozniak, M.A., Mee, A.P., and Itzhaki, R.F. (2009b). Herpes simplex virus type 1 DNA is located within Alzheimer’s disease amyloid plaques. J Pathol 217, 131–138.
Wu, G.D., Chen, J., Hoffmann, C., Bittinger, K., Chen, Y.Y., Keilbaugh, S.A., Bewtra, M., Knights, D., Walters, W.A., Knight, R., Sinha, R., Gilroy, E., Gupta, K., Baldassano, R., Nessel, L., Li, H.Z., Bushman, F.D., and Lewis, J.D. (2011). Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108.
Yamada, T., Kadekaru, H., Matsumoto, S., Inada, H., Tanabe, M., Moriguchi, E.H., Moriguchi, Y., Ishikawa, P., Ishikawa, A.G., Taira, K., and Yamori, Y. (2002). Prevalence of dementia in the older Japanese-Brazilian population. Psychiatry Clin Neurosci 56, 71–75.
Yang, T., Santisteban, M.M., Rodriguez, V., Li, E., Ahmari, N., Carvajal, J.M., Zadeh, M., Gong, M., Qi, Y., Zubcevic, J., Sahay, B., Pepine, C.J., Raizada, M.K., and Mohamadzadeh, M. (2015). Gut dysbiosis is linked to hypertension. Hypertension 65, 1331–1340.
Yano, J.M., Yu, K., Donaldson, G.P., Shastri, G.G., Ann, P., Ma, L., Nagler, C.R., Ismagilov, R.F., Mazmanian, S.K., and Hsiao, E.Y. (2015). Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161, 264–276.
Yehuda, S., Rabinovitz, S., and Mostofsky, D.I. (2005). Essential fatty acids and the brain: from infancy to aging. Neurobiol Aging 26, 98–102.
Yu, H.N., Zhu, J., Pan, W.S., Shen, S.R., Shan, W.G., and Das, U.N. (2014). Effects of fish oil with a high content of n-3 polyunsaturated fatty acids on mouse gut microbiota. Arch Med Res 45, 195–202.
Zandi, P.P., Anthony, J.C., Khachaturian, A.S., Stone, S.V., Gustafson, D., Tschanz, J.T., Norton, M.C., Welsh-Bohmer, K.A., Breitner, J.C.S., and Grp, C.C.S. (2004). Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: The Cache County Study. Arch Neurol 61, 82–88.
Zhang, C., Li, S., Yang, L., Huang, P., Li, W., Wang, S., Zhao, G., Zhang, M., Pang, X., Yan, Z., Liu, Y., and Zhao, L. (2013). Structural modulation of gut microbiota in life-long calorie-restricted mice. Nat Commun 4, 2163.
Zhang, R., Miller, R.G., Gascon, R., Champion, S., Katz, J., Lancero, M., Narvaez, A., Honrada, R., Ruvalcaba, D., and McGrath, M.S. (2009). Circulating endotoxin and systemic immune activation in sporadic amyotrophic lateral sclerosis (sALS). J Neuroimmunol 206, 121–124.
Ziegler-Graham, K., Brookmeyer, R., Johnson, E., and Arrighi, H.M. (2008). Worldwide variation in the doubling time of Alzheimer’s disease incidence rates. Alzheimers Dementia 4, 316–323.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published with open access at link.springer.com
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hu, X., Wang, T. & Jin, F. Alzheimer’s disease and gut microbiota. Sci. China Life Sci. 59, 1006–1023 (2016). https://doi.org/10.1007/s11427-016-5083-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-016-5083-9