Abstract
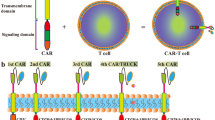
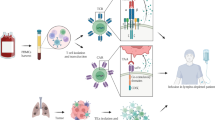
The successes achieved by chimeric antigen receptor-modified T (CAR-T) cells in hematological malignancies raised the possibility of their use in non-small lung cancer (NSCLC). In this phase I clinical study (NCT01869166), patients with epidermal growth factor receptor (EGFR)-positive (>50% expression), relapsed/refractory NSCLC received escalating doses of EGFR-targeted CAR-T cell infusions. The EGFR-targeted CAR-T cells were generated from peripheral blood after a 10 to 13-day in vitro expansion. Serum cytokines in peripheral blood and copy numbers of CAR-EGFR transgene in peripheral blood and in tissue biopsy were monitored periodically. Clinical responses were evaluated with RECIST1.1 and immune- related response criteria, and adverse events were graded with CTCAE 4.0. The EGFR-targeted CAR-T cell infusions were well-tolerated without severe toxicity. Of 11 evaluable patients, two patients obtained partial response and five had stable disease for two to eight months. The median dose of transfused CAR+ T cells was 0.97×107 cells kg−1 (interquartile range (IQR), 0.45 to 1.09×107 cells kg−1). Pathological eradication of EGFR positive tumor cells after EGFR-targeted CAR-T cell treatment can be observed in tumor biopsies, along with the CAR-EGFR gene detected in tumor-infiltrating T cells in all four biopsied patients. The EGFR-targeted CAR-T cell therapy is safe and feasible for EGFR-positive advanced relapsed/refractory NSCLC.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Ahmed, N., Brawley, V.S., Hegde, M., Robertson, C., Ghazi, A., Gerken, C., Liu, E., Dakhova, O., Ashoori, A., Corder, A., Gray, T., Wu M.F., Liu, H., Hicks, J., Rainusso, N., Dotti, G., Mei, Z., Grilley, B., Gee A., Rooney, C.M., Brenner, M.K., Heslop, H.E., Wels, W.S., Wang, L.L., Anderson, P., and Gottschalk, S. (2015). Human epidermal growth factor receptor 2 (HER2)-specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-positive sarcoma. J Clin Oncol 33, 1688–1696.
Alizadeh, D., and Larmonier, N. (2014). Chemotherapeutic targeting of cancer-induced immunosuppressive cells. Cancer Res 74, 2663–2668.
American Cancer Society (2013). Cancer Facts & Figures 2013 (Atlanta: American Cancer Society).
Anraku, M., Tagawa, T., Wu, L., Yun, Z., Keshavjee, S., Zhang, L., Johnston, M.R., and De Perrot, M. (2010). Synergistic antitumor effects of regulatory T cell blockade combined with pemetrexed in murine malignant mesothelioma. J Immunol 185, 956–966.
Azzoli, C.G., Baker, S. J., Temin, S., Pao, W., Aliff, T., Brahmer, J., Johnson, D.H., Laskin, J.L., Masters, G., Milton, D., Nordquist, L., Pfister, D.G., Piantadosi, S., Schiller, J.H., Smith, R., Smith, T.J., Strawn, J.R., Trent, D., Giaccone, G., (2009). American society of clinical oncology clinical practice guideline update on chemotherapy for stage IVnon-small-cell lung cancer. J Clin Oncol 27, 6251–6266.
Brentjens, R.J., Davila, M.L., Riviere, I., Park, J., Wang, X., Cowell, L.G., Bartido, S., Stefanski, J., Taylor, C., Olszewska, M., Borquez-Ojeda, O., Qu, J., Wasielewska, T., He, Q., Bernal, Y., Rijo, I.V., Hedvat, C., Kobos, R., Curran, K., Steinherz, P., Jurcic, J., Rosenblat, T., Maslak, P., Frattini, M., and Sadelain, M. (2013). CD19-Targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 5, 177ra38.
Bunn, P.J. (2002). Chemotherapy for advanced non-small-cell lung cancer: who, what, when, why? J Clin Oncol 20(18 Suppl), 23S–33S.
Caruso, H.G., Hurton, L.V., Najjar, A., Rushworth, D., Ang, S., Olivares, S., Mi, T., Switzer, K., Singh, H., Huls, H., Lee, D.A., Heimberger, A.B., Champlin, R.E., and Cooper, L.J. (2015). Tuning sensitivity of CAR to EGFR density limits recognition of normal tissue while maintaining potent antitumor activity. Cancer Res 75, 3505–3518.
Chmielewski, M., Hombach, A., Heuser, C., Adams, G.P., and Abken, H. (2014). T cell activation by antibody-like immunoreceptors: increase in affinity of the single-chain fragment domain above threshold does not increase T cell activation against antigen-positive target cells but decreases selectivity. J Immunol 173, 7647–7653.
Ciardiello, F., and Tortora, G. (2008). EGFR antagonists in cancer treatment. N Engl J Med 358, 1160–1174.
D’Addario, G., and Felip, E. (2009). Non-small-cell lung cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 20(4 Suppl), 68–70.
Dudley, M.E., Wunderlich, J.R., Robbins, P.F., Yang, J.C., Hwu, P., Schwartzentruber, D.J., Topalian, S.L., Sherry, R., Restifo, N.P., Hubicki, A.M., Robinson, M.R., Raffeld, M., Duray, P., Seipp, C.A., Rogers-Freezer, L., Morton, K.E., Mavroukakis, S.A., White, D.E., and Rosenberg, S.A. (2002). Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 298, 850–854.
Eisenhauer, E.A., Therasse, P., Bogaerts, J., Schwartz, L.H., Sargent, D., Ford, R., Dancey, J., Arbuck, S., Gwyther, S., Mooney, M., Rubinstein, L., Shankar, L., Dodd, L., Kaplan, R., Lacombe, D., and Verweij, J. (2009). New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1). Eur J Cancer 45, 228–247.
Eshhar, Z., Waks, T., Gross, G., and Schindler, D.G. (1993). Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci USA 90, 720–724.
Garnett, C.T., Schlom, J., and Hodge, J.W. (2008). Combination of docetaxel and recombinant vaccine enhances T-cell responses and antitumoractivity: effects of docetaxel on immune enhancement. Clin Cancer Res 14, 3536–3544.
Gatzemeier, U., Pluzanska, A., Szczesna, A., Kaukel, E., Roubec, J., De Rosa, F., Milanowski, J., Karnicka-Mlodkowski, H., Pesek, M., Serwatowski, P., Ramlau, R., Janaskova, T., Vansteenkiste, J., Strausz, J., Manikhas, G.M., and von Pawel, J. (2007). Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol 25, 1545–1552.
Giaccone, G., Herbst, R.S., Manegold, C., Scagliotti, G., Rosell, R., Miller, V., Natale R.B., Schiller, J.H., von Pawel, J., Pluzanska, A., Gatzemeier, U., Grous, J., Ochs, J.S., Averbuch, S.D., Wolf, M.K., Rennie, P., Fandi, A., and Johnson, D.H. (2004). Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial—INTACT 1. J Clin Oncol 22, 777–784.
Grossi, F., Aita, M., Defferrari, C., Rosetti, F., Brianti, A., Fasola, G., Vinante, O., Pronzato, P., and Pappagallo, G. (2009). Impact of third-generation drugs on the activity of first-line chemotherapy in advanced non-small cell lung cancer: a meta-analytical approach. Oncologist 14, 497–510.
Grupp, S.A., Kalos, M., Barrett, D., Aplenc, R., Porter, D.L., Rheingold, S.R., Teachey, D.T., Chew, A., Hauck, B., Wright, J.F., Milone, M.C., Levine, B.L., and June, C.H. (2013). Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 368, 1509–1518.
Herbst, R.S., Giaccone, G., Schiller, J.H., Natale, R.B., Miller, V., Manegold, C., Scagliotti, G., Rosell R, Oliff, I., Reeves, J.A., Wolf, M.K., Krebs, A.D., Averbuch, S.D., Ochs, J.S., Grous, J., Fandi, A., and Johnson, D.H. (2004). Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial— INTACT 2. J Clin Oncol 22, 785–794.
Herbst, R.S., Prager, D., Hermann, R., Fehrenbacher, L., Johnson, B.E., Sandler, A., Kris, M.G., Tran, H.T., Klein, P., Li, X., Ramies, D., Johnson, D.H., and Miller, V.A. (2005). TRIBUTE: a phase III trial of Erlotinib Hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 23, 5892–5899.
Kang, T.H., Mao, C.P., Lee, S.Y., Chen, A., Lee, J.H., Kim, T.W., Alvarez, R.D., Roden, R.B., Pardoll, D., Hung, C.F., and Wu, T.C. (2013). Chemotherapy acts as an adjuvant to convert the tumor microenvironment into a highly permissive state for vaccination-induced antitumor immunity. Cancer Res 73, 2493–2504.
Kershaw, M.H., Devaud, C., John, L.B., Westwood, J.A., and Darcy, P.K. (2013). Enhancing immunotherapy using chemotherapy and radiation to modify the tumor microenvironment. Oncoimmunology 2, e25962.
Kochenderfer, J.N., Dudley, M.E., Feldman, S.A., Wilson,W.H., Spaner, D.E., Maric, I., Stetler-Stevenson, M., Phan, G.Q., Hughes, M.S., Sherry, R.M., Yang, J.C., Kammula, U.S., Devillier, L., Carpenter, R., Nathan, D.A., Morgan, R.A., Laurencot, C., and Rosenberg, S.A. (2012). B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 119, 2709–2720.
Kochenderfer, J.N., Dudley, M.E., Kassim, S.H., Somerville, R.P., Carpenter, R.O., Stetler-Stevenson, M., Yang, J.C., Phan, G.Q., Hughes, M.S., Sherry, R.M., Raffeld, M., Feldman, S., Lu, L., Li, Y.F., Ngo, L.T., Goy, A., Feldman, T., Spaner, D.E., Wang, M.L., Chen, C.C., Kranick, S.M., Nath, A., Nathan, D.A., Morton, K.E., Toomey, M.A., and Rosenberg, S.A. (2015). Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol 33, 540–549.
Li, Y., Yin, J., Li, T., Huang, S., Yan, H., Leavenworth, J., and Wang, X. (2015). NK cell-based cancer immunotherapy: from basic biology to clinical application. Sci China Life Sci 58, 1233–1245.
Liu, X., Jiang, S., Fang, C., Yang S., Olalere, D., Pequignot, E.C., Cogdill, A.P., Li, N., Ramones, M., Granda, B., Zhou, L., Loew, A., Young, R.M., June, C.H., and Zhao, Y. (2015). Affinity-tuned ErbB2 or EGFR chimeric antigen receptor T cells exhibit an increased therapeutic index against tumors in mice. Cancer Res 75, 3596–3607.
Louis, C.U., Savoldo, B., Dotti, G., Pule, M., Yvon, E., Myers, G.D., Rossig, C., Russell, H.V., Diouf, O., Liu, E., Liu, H., Wu, M.F., Gee, A.P., Mei, Z., Rooney, C.M., Heslop, H.E., and Brenner, M.K. (2011). Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 118, 6050–6056.
McGinley, L., McMahon, J., Strappe, P., Barry, F., Murphy, M., O’Toole, D., O’Brien, T. (2011). Lentiviral vector mediated modification of mesenchymal stem cells & enhanced survival in an in vitro model of ischaemia. Stem Cell Res Ther 2, 12.
Morgan, R.A., Yang, J.C., Kitano, M., Dudley, M.E., Laurencot, C.M., and Rosenberg, S.A. (2010). Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther 18, 843–851.
Muranski, P., Boni A., Wrzesinski, C., Citrin, D.E., Rosenberg, S.A., Childs, R., and Restifo, N.P. (2006). Increased intensity lymphodepletion and adoptive immunotherapy—how far can we go? Nat Clin Pract Oncol 3, 668–681.
Oxnard, G.R., Arcila, M.E., Chmielecki, J., Ladanyi, M., Miller, V.A., and Pao, W. (2011). New strategies in overcoming acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitorsin lung cancer. Clin Cancer Res 17, 5530–5537.
Porter, D.L., Levine, B.L., Kalos, M., Baqq, A., and June, C.H. (2011). Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 365, 725–733.
Robbins, P.F., Dudley, M.E., Wunderlich, J., El-Gamil, M., Li, Y.F., Zhou, J., Huang, J., Powell, D.J., and Rosenberg, S.A. (2004). Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immun 173, 7125–7130.
Robbins, P.F., Morgan, R.A., Feldman, S.A., Yang, J.C., Sherry, RM., Dudley, M.E., Wunderlich, J.R., Nahvi, A.V., Helman, L.J., Mackall, C.L., Kammula, U.S., Hughes, M.S., Restifo, N.P., Raffeld, M., Lee, C.C., Levy, C.L., Li, Y.F., El-Gamil, M., Schwarz, S.L., Laurencot, C., and Rosenberg, S.A. (2011). Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol 29, 917–924.
Salomon, D.S., Brandt, R., Ciardiello, F., and Normanno, N. (1995). Epidermal growth factor related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol 19, 183–232.
Savoldo, B., Ramos, C.A., Liu, E., Mims, M.P., Keating, M.J., Carrum, G., Kamble, R.T., Bollard, C.M., Gee, A.P., Mei, Z., Liu, H., Grilley, B., Rooney, C.M., Heslop, H.E., Brenner, M.K., and Dotti, G. (2001). CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest 121, 1822–1826.
Scagliotti, G.V., Parikh, P., von Pawel, J., Biesma, B., Vansteenkiste, J., Manegold, C., Serwatowski, P., Gatzemeier, U., Digumarti, R., Zukin, M., Lee, J.S., Mellemgaard, A., Park, K., Patil, S., Rolski, J., Goksel, T., De Marinis, F., Simms, L., Sugarman, K.P., and Gandara, D. (2008). Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small cell lung cancer. J Clin Oncol 26, 3543–3551.
Till, B.G., Jensen, M.C., Wang, J., Chen, E.Y., Wood, B.L., Greisman, H.A., Qian, X., James, S.E., Raubitschek, A., Forman, S.J., Gopal, A.K., Pagel, J.M., Lindgren, C.G., Greenberg, P.D., Riddell, S.R., and Press, O.W. (2008). Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood 112, 2261–2271.
Till, B.G., Jensen, M.C., Wang, J., Qian, X., Gopal, A.K., Maloney, D.G., Lindgren, C.G., Lin, Y., Pagel, J.M., Budde, L.E., Raubitschek, A., Forman, S.J., Greenberg, P.D., Riddell, S.R., and Press, O.W. (2012). CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood 119, 3940–3950.
Wang, Y., Dai, H., Li, H., Lv, H.Y., Wang, T., Fu, X., and Han, W. (2011). Growth of Human colorectal cancer SW1116 cells is inhibited by cytokine-induced killer cells. Clin Dev Immunol 2011, doi: 10.1155/2011/621414.
Wolchok, J.D., Hoos, A., O’Day, S., Weber, J.S., Hamid, O., Lebbé, C., Maio, M., Binder, M., Bohnsack, O., Nichol, G., Humphrey, R., and Hodi, F.S. (2009). Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 15, 7412–7420.
Yamada, T., Azuma, K., Muta, E., Kim, J., Suqawara, S., Zhang, G.L., Matsueda, S., Kasama-Kawaguchi, Y., Yamashita, Y., Yamashita, T., Nishio, K., Itoh, K., Hoshino, T., and Sasada, T. (2013). EGFR T790M mutation as a possible target for immunotherapy; identification of HLA-A *0201-restricted T cell epitopes derived from the EGFR T790M mutation. PLoS One 8, e78389.
Zheng, Z., Chinnasamy, N., and Morgan, R.A. (2012). Protein L: a novel reagent for the detection of chimeric antigen receptor (CAR) expression by flow cytometry. J Transl Med 10, 29.
Zhou, X., Li, J., Wang, Z., Chen, Z., Qiu, J., Zhang, Y., Wang, W., Ma, Y., Huang, N., Cui, K., Li, J., and Wei, Y.Q. (2013). Cellular immunotherapy for carcinoma using genetically modified EGFR-specific T lymphocytes. Neoplasia 15, 544–553.
Author information
Authors and Affiliations
Corresponding author
Additional information
Contributed equally to this work
This article is published with open access at link.springer.com
Electronic supplementary material
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Feng, K., Guo, Y., Dai, H. et al. Chimeric antigen receptor-modified T cells for the immunotherapy of patients with EGFR-expressing advanced relapsed/refractory non-small cell lung cancer. Sci. China Life Sci. 59, 468–479 (2016). https://doi.org/10.1007/s11427-016-5023-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-016-5023-8