Abstract
Purpose
One of the ongoing research subjects for forensic analysts is differentiating halogen positional isomers of newly-emerging synthetic cannabinoids. The purpose of this study is to elucidate liquid chromatographic and mass spectrometric conditions applicable to the differentiation of such derivatives.
Methods
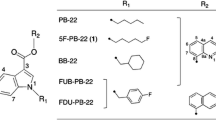
High-performance liquid chromatography (HPLC) coupled with triple quadrupole mass spectrometry (QqQ-MS) and linear ion trap time-of-flight mass spectrometry (IT-TOF-MS) using electrospray ionization (ESI) in its positive ion mode were utilized to analyze six model compounds, FUB-JWH-018 and five positional isomers having structures of 1- or 2-naphthoyl-substituted 1H-indole-3-carboxylates with N-substituted positional isomeric fluorobenzyl groups (2-fluorobenzyl, 3-fluorobenzyl, and 4-fluorobenzyl).
Results
The chromatographic separation of the six isomers was successfully achieved by HPLC using a pentafluorophenylpropyl-bonded reversed-phase adsorbent. The positive ESI-QqQ-MS could discriminate fluorobenzyl isomers having a same naphthoyl structure via the relative abundance of the two product ions in the collision-induced dissociation reaction. ESI-IT-TOF-MS in its positive ion mode successfully distinguished three ring positional isomers in both naphthoyl scaffolds on the basis of the differences in the abundance of oxomethylium ion attributed to C16H11FNO+ (m/z 252).
Conclusions
The use of ESI-QqQ-MS and ESI-IT-TOF-MS in its positive ion mode coupled with LC using a pentafluorophenylpropyl-bonded silica column is applicable to MS-aided differentiation and the chromatographic separation of FUB-JWH-018 positional isomers.






Similar content being viewed by others
References
Kikura-Hanajiri R, Uchiyama N, Kawamura M, Ogata J, Goda Y (2013) Prevalence of new designer drugs and their legal status in Japan. Yakugaku Zasshi 133:31–40 (in Japanese with English abstract)
Underwood E (2015) A new drug war. Science 347:469–473
Kikura-Hanajiri R (2016) New designer drugs in Japan. In: Victor RP (ed) Neuropathology of drug addictions and substance misuse, vol 2. Academic Press, London, pp 1055–1065
Bijlsma L, Sancho JV, Hernandez F, Niessen WM (2011) Fragmentation pathways of drugs of abuse and their metabolites based on QTOF MS/MS and MSE accurate-mass spectra. J Mass Spectrom 46:865–875
Zaitsu K, Miyagawa H, Sakamoto Y, Matsuta S, Tsuboi K, Nishioka H, Katagi M, Sato T, Tatsuno M, Tsuchihashi H, Suzuki K, Ishii A (2013) Mass spectrometric differentiation of the isomers of mono-methoxyethylamphetamines and mono-methoxydimethylamphetamines by GC–EI–MS–MS. Forensic Toxicol 31:292–300
Kusano M, Zaitsu K, Nakayama H, Nakajima J, Hisatsune K, Moriyasu T, Matsuta S, Katagi M, Tsuchihashi H, Ishii A (2015) Positional isomer differentiation of synthetic cannabinoid JWH-081 by GC-MS/MS. J Mass Spectrom 50:586–591
Kusano M, Yamanaka M, Zaitsu K, Nakayama H, Nakajima J, Moriyasu T, Tsuchihashi H, Ishii A (2016) Regioisomeric differentiation of the alkyl-substituted synthetic cannabinoids JWH-122 and JWH-210 by GC-EI-MS/MS. Forensic Toxicol 34:304–315
DeRuiter J, Smith FT, Abdel-Hay K, Clark CR (2014) Analytical differentiation of 1-alkyl-3-acylindoles and 1-acyl-3-alkylindoles: isomeric synthetic cannabinoids. Anal Chem 86:3801–3808
Abdel-Hay KM, De Ruiter J, Smith F, Alsegiani AS, Thaxton-Weissenfluh A, Clark CR (2016) GC–MS differentiation of the six regioisomeric dimethoxybenzoyl-1-pentylindoles: isomeric cannabinoid substances. J Pharm Biomed Anal 125:360–368
Westphal F, Junge T (2012) Ring positional differentiation of isomeric N-alkylated fluorocathinones by gas chromatography/tandem mass spectrometry. Forensic Sci Int 223:97–105
Negishi S, Nakazono Y, Iwata YT, Kanamori T, Tsujikawa K, Kuwayama K, Yamamuro T, Miyamoto K, Yamashita T, Kasuya F, Inoue H (2015) Differentiation of regioisomeric chloroamphetamine analogs using gas chromatography–chemical ionization-tandem mass spectrometry. Forensic Toxicol 33:338–347
Murakami T, Iwamuro Y, Ishimaru R, Chinaka S, Sugimura N, Takayama N (2016) Differentiation of AB-FUBINACA positional isomers by the abundance of product ions using electron ionization-triple quadrupole mass spectrometry. J Mass Spectrom 51:1016–1022
Kohyama E, Chikumoto T, Tada H, Kitaichi K, Ito T (2017) Analytical differentiation of quinolinyl- and isoquinolinyl-substituted 1-(5-fluoropentyl)-1H-indole-3-carboxylates: 5F-PB-22 and its ten isomers. Forensic Toxicol 35:56–65
Murakami T, Iwamuro Y, Ishimaru R, Chinaka S, Noda I, Higashibayashi S, Takayama N (2017) Elucidation of the fluorine substitution position on the phenyl ring of synthetic cannabinoids by electron ionization–triple quadrupole mass spectrometry. Jpn J Forensic Sci Tech 22:133–143
Murakami T, Iwamuro Y, Ishimaru R, Chinaka S, Takayama N, Hasegawa H (2018) Differentiation of AB-FUBINACA and its five positional isomers using liquid chromatography–electrospray ionization-linear ion trap mass spectrometry and triple quadrupole mass spectrometry. Forensic Toxicol 36:351–358
Kohyama E, Chikumoto T, Furukawa R, Suenami K, Kawashima H, Tada H, Nagai H, Soda M, Kitaichi K, Ito T (2017) Regioisomeric differentiation of synthetic cannabinoids with an N-fluorobenzyl indole core by gas chromatography–tandem mass spectrometry. Forensic Chem 6:28–35
Shizuoka Prefectural Government (2015) Media release, 25th November. http://www2.pref.shizuoka.jp/all/kisha15.nsf/c3db48f94231df2e4925714700049a4e/dadcf8e168630c7849257ef300270c57?OpenDocument. Accessed Apr 2018
Wohlfarth A, Gandhi AS, Pang S, Zhu M, Scheidweiler KB, Huestis MA (2014) Metabolism of synthetic cannabinoids PB-22 and its 5-fluoro analog, 5F-PB-22, by human hepatocyte incubation and high-resolution mass spectrometry. Anal Bioanal Chem 406:1763–1780
Kevin RC, Lefever TW, Snyder RW, Patel PR, Fennell TR, Wiley JL, McGregor IS, Thomas BF (2017) In vitro and in vivo pharmacokinetics and metabolism of synthetic cannabinoids CUMYL-PICA and 5F-CUMYL-PICA. Forensic Toxicol 35:333–347
Acknowledgements
This work was funded by the domestically programmed Grant for the regional society from the Gifu Prefectural Research Institute for Health and Environmental Sciences. The study was supported by the Health and Labour Sciences Research Grants 2015 and 2016 to K. Kitaichi (Research on Regulatory Science of Pharmaceuticals and Medical Devices, No. 27170401). A portion of this work was supported by the governmental program on a survey of designer drugs in the illegal drug market, supervised by Gifu Prefectural Government, Japan. The study was also supported by the Grants for research on regional health, medical, and welfare to T. Ito (The Daido Life Welfare Foundation). We are thankful to Shizuoka Prefectural Government, Japan, for providing the two illegal herbal products for inspection. We acknowledge the Gifu Regional Consortium on the Development of Analytical Procedures for Legal Highs operated by Gifu Pharmaceutical University and the Gifu Prefectural Research Institute for Health and Environmental Sciences for overseeing the experimental protocols.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There are no financial or other relations that could lead to a conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chikumoto, T., Furukawa, R., Kohyama, E. et al. Liquid chromatography–mass spectrometry studies on the isomeric 1-fluorobenzyl-3-naphthoyl-indoles: FUB-JWH-018 and five isomers. Forensic Toxicol 37, 113–120 (2019). https://doi.org/10.1007/s11419-018-0442-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11419-018-0442-9