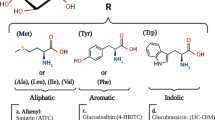
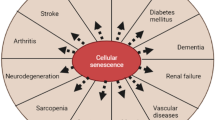
Abstract
In the last several years, numerous molecules derived from plants and vegetables have been tested for their antioxidant, anti-inflammatory, and anti-aging properties. One of them is sulforaphane (SFN), an isothiocyanate present in cruciferous vegetables. SFN activates the antioxidant and anti-inflammatory responses by inducing Nrf2 pathway and inhibiting NF-κB. It also has an epigenetic effect by inhibiting HDAC and DNA methyltransferases and modifies mitochondrial dynamics. Moreover, SFN preserves proteome homeostasis (proteostasis) by activating the proteasome, which has been shown to lead to increased cellular lifespan and prevent neurodegeneration. In this review, we describe some of the molecular and physical characteristics of SFN, its mechanisms of action, and the effects that SFN treatment induces in order to discuss its relevance as a “miraculous” drug to prevent aging and neurodegeneration.






Similar content being viewed by others
References
Alfieri A, Srivastava S, Siow RC, Cash D, Modo M, Duchen MR, … Mann GE (2013) Sulforaphane preconditioning of the Nrf2/HO-1 defense pathway protects the cerebral vasculature against blood–brain barrier disruption and neurological deficits in stroke. Free Radic Biol Med 65:1012–1022
Angelino D, Jeffery E (2014) Glucosinolate hydrolysis and bioavailability of resulting isothiocyanates: focus on glucoraphanin. J Funct Foods 7:67–76
Atwell LL, Hsu A, Wong CP, Stevens JF, Bella D, Yu TW, … Dashwood RH (2015) Absorption and chemopreventive targets of sulforaphane in humans following consumption of broccoli sprouts or a myrosinase-treated broccoli sprout extract. Mol Nutr Food Res 59(3):424–433
Bai Y, Cui W, Xin Y, Miao X, Barati MT, Zhang C et al (2013) Prevention by sulforaphane of diabetic cardiomyopathy is associated with up-regulation of Nrf2 expression and transcription activation. J Mol Cell Cardiol 57:82–95
Barba FJ, Nikmaram N, Roohinejad S, Khelfa A, Zhu Z, Koubaa M (2016) Bioavailability of glucosinolates and their breakdown products: impact of processing. Front Nutr 3:24
Bhakkiyalakshmi E, Sireesh D, Rajaguru P, Paulmurugan R, Ramkumar KM (2015) The emerging role of redox-sensitive Nrf2–Keap1 pathway in diabetes. Pharmacol Res 91:104–114
Bheemreddy RM, Jeffery EH (2007) The metabolic fate of purified glucoraphanin in F344 rats. J Agric Food Chem 55(8):2861–2866
Bones AM, Rossiter JT (2006) The enzymic and chemically induced decomposition of glucosinolates. Phytochemistry 67(11):1053–1067
Bricker GV, Riedl KM, Ralston RA, Tober KL, Oberyszyn TM, Schwartz SJ (2014) Isothiocyanate metabolism, distribution, and interconversion in mice following consumption of thermally processed broccoli sprouts or purified sulforaphane. Mol Nutr Food Res 58(10):1991–2000
Brown AF, Yousef GG, Jeffery EH, Klein BP, Wallig MA, Kushad MM, Juvik JA (2002) Glucosinolate profiles in broccoli: variation in levels and implications in breeding for cancer chemoprotection. J Am Soc Hortic Sci 127(5):807–813
Budenholzer L, Cheng CL, Li Y, Hochstrasser M (2017) Proteasome structure and assembly. J Mol Biol 429(22):3500–3524
Burmeister WP, Cottaz S, Rollin P, Vasella A, Henrissat B (2000) High resolution X-ray crystallography shows that ascorbate is a cofactor for myrosinase and substitutes for the function of the catalytic base. J Biol Chem 275:39385–39393
Campisi J, di Fagagna F d A (2007) Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 8(9):729–740
Chapple SJ, Siow RC, Mann GE (2012) Crosstalk between Nrf2 and the proteasome: therapeutic potential of Nrf2 inducers in vascular disease and aging. Int J Biochem Cell Biol 44(8):1315–1320
Checker R, Gambhir L, Thoh M, Sharma D, Sandur SK (2015) Sulforaphane, a naturally occurring isothiocyanate, exhibits anti-inflammatory effects by targeting GSK3β/Nrf-2 and NF-κB pathways in T cells. J Funct Foods 19:426–438
Childs BG, Durik M, Baker DJ, Van Deursen JM (2015) Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med 21(12):1424–1435
Chondrogianni N, Gonos ES (2007) Overexpression of hUMP1/POMP proteasome accessory protein enhances proteasome-mediated antioxidant defence. Exp Gerontol 42(9):899–903
Chondrogianni N, Petropoulos I, Franceschi C, Friguet B, Gonos ES (2000) Fibroblast cultures from healthy centenarians have an active proteasome. Exp Gerontol 35(6–7):721–728
Chondrogianni N, Stratford FL, Trougakos IP, Friguet B, Rivett AJ, Gonos ES (2003) Central role of the proteasome in senescence and survival of human fibroblasts: induction of a senescence-like phenotype upon its inhibition and resistance to stress upon its activation. J Biol Chem 278:28026–28037
Chondrogianni N, Tzavelas C, Pemberton AJ, Nezis IP, Rivett AJ, Gonos ES (2005) Overexpression of proteasome β5 subunit increases amount of assembled proteasome and confers ameliorated response to oxidative stress and higher survival rates. J Biol Chem 280:11840–11850
Chondrogianni N, Trougakos IP, Kletsas D, Chen QM, Gonos ES (2008) Partial proteasome inhibition in human fibroblasts triggers accelerated M1 senescence or M2 crisis depending on p53 and Rb status. Aging Cell 7(5):717–732
Chondrogianni N, Voutetakis K, Kapetanou M, Delitsikou V, Papaevgeniou N, Sakellari M, Lefaki M, Filippopoulou K, Gonos ES (2015) Proteasome activation: an innovative promising approach for delaying aging and retarding age-related diseases. Ageing Res Rev 23:37–55
Chondrogianni N, Georgila K, Kourtis N, Tavernarakis N, Gonos ES (2015a) 20S proteasome activation promotes life span extension and resistance to proteotoxicity in Caenorhabditis elegans. FASEB J 29(2):611–622
Chondrogianni N, Sakellari M, Lefaki M, Papaevgeniou N, Gonos ES (2015b) Proteasome activation delays aging in vitro and in vivo. Free Radic Biol Med 71:303–320
Ciechanover A (1998) The ubiquitin–proteasome pathway: on protein death and cell life. EMBO J 17(24):7151–7160
Clarke JD, Hsu A, Williams DE, Dashwood RH, Stevens JF, Yamamoto M, Ho E (2011) Metabolism and tissue distribution of sulforaphane in Nrf2 knockout and wild-type mice. Pharm Res 28(12):3171–3179
Colditz GA, Branch LG, Lipnick RJ, Willett WC, Rosner B, Posner BM, Hennekens CH (1985) Increased green and yellow vegetable intake and lowered cancer deaths in an elderly population. Am J Clin Nutr 41(1):32–36. https://doi.org/10.1093/ajcn/41.1.32
Cramer JM, Jeffery EH (2011) Sulforaphane absorption and excretion following ingestion of a semi-purified broccoli powder rich in glucoraphanin and broccoli sprouts in healthy men. Nutr Cancer 63(2):196–201
Dai D-F, Chiao YA, Marcinek DJ, Szeto HH, Rabinovitch PS (2014) Mitochondrial oxidative stress in aging and healthspan. Longev Healthspan 3(1):6
Daniel M, Tollefsbol TO (2015) Epigenetic linkage of aging, cancer and nutrition. J Exp Biol 218(1):59–70
Dashwood RH, Ho E (2008) Dietary agents as histone deacetylase inhibitors: sulforaphane and structurally related isothiocyanates. Nutr Rev 66:S36–S38
Dinkova-Kostova AT, Fahey JW, Kostov RV, Kensler TW (2017) KEAP1 and done? Targeting the NRF2 pathway with sulforaphane. Trends Food Sci Technol 69:257–269
Dwivedi S, Rajasekar N, Hanif K, Nath C, Shukla R (2016) Sulforaphane ameliorates okadaic acid-induced memory impairment in rats by activating the Nrf2/HO-1 antioxidant pathway. Mol Neurobiol 53(8):5310–5323
Fahey J, Talalay P (1999) Antioxidant functions of sulforaphane: a potent inducer of phase II detoxication enzymes. Food Chem Toxicol 37(9–10):973–979
Fishbein JC, Heilman JM (2018) Advances in molecular toxicology. Elsevier Science
Gabriel D, Roedl D, Gordon LB, Djabali K (2015) Sulforaphane enhances progerin clearance in Hutchinson–Gilford progeria fibroblasts. Aging Cell 14(1):78–91
Gan N, Wu Y-C, Brunet M, Garrido C, Chung F-L, Dai C, Mi L (2010) Sulforaphane activates heat shock response and enhances proteasome activity through up-regulation of Hsp27. J Biol Chem 285(46):35528–35536
Gao J, Xiong B, Zhang B, Li S, Huang N, Zhan G et al (2018) Sulforaphane alleviates lipopolysaccharide-induced spatial learning and memory dysfunction in mice: the role of BDNF-mTOR signaling pathway. Neuroscience 388:357–366
Ghawi SK, Methven L, Niranjan K (2013) The potential to intensify sulforaphane formation in cooked broccoli (Brassica oleracea var. italica) using mustard seeds (Sinapis alba). Food Chem 138(2–3):1734–1741
Gordon LB, Rothman FG, López-Otín C, Misteli T (2014) Progeria: a paradigm for translational medicine. Cell 156(3):400–407
Greco T, Shafer J, Fiskum G (2011) Sulforaphane inhibits mitochondrial permeability transition and oxidative stress. Free Radic Biol Med 51(12):2164–2171
Grünwald S, Stellzig J, Adam IV, Weber K, Binger S, Boll M, Knorr E, Twyman RM, Vilcinskas A, Wenzel U (2013) Longevity in the red flour beetle Tribolium castaneum is enhanced by broccoli and depends on nrf-2, jnk-1 and foxo-1 homologous genes. Genes Nutr 8(5):439–448
Guerrero-Beltrán CE, Calderón-Oliver M, Pedraza-Chaverri J, Chirino YI (2012) Protective effect of sulforaphane against oxidative stress: recent advances. Exp Toxicol Pathol 64(5):503–508
Guo L, Yang R, Wang Z, Guo Q, Gu Z (2014) Glucoraphanin, sulforaphane and myrosinase activity in germinating broccoli sprouts as affected by growth temperature and plant organs. J Funct Foods 9:70–77
Hanlon N, Coldham N, Gielbert A, Kuhnert N, Sauer MJ, King LJ, Ioannides C (2008) Absolute bioavailability and dose-dependent pharmacokinetic behaviour of dietary doses of the chemopreventive isothiocyanate sulforaphane in rat. Br J Nutr 99(3):559–564
Hariton F, Xue M, Rabbani N, Fowler M, Thornalley PJ (2018) Sulforaphane delays fibroblast senescence by curbing cellular glucose uptake, increased glycolysis, and oxidative damage. Oxidative Med Cell Longev
Heiss E, Herhaus C, Klimo K, Bartsch H, Gerhauser C (2001) Nuclear factor-κB is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J Biol Chem 276:32008–32015
Hernandez-Rabaza V, Cabrera-Pastor A, Taoro-Gonzalez L, Gonzalez-Usano A, Agusti A, Balzano T et al (2016) Neuroinflammation increases GABAergic tone and impairs cognitive and motor function in hyperammonemia by increasing GAT-3 membrane expression. Reversal by sulforaphane by promoting M2 polarization of microglia. J Neuroinflammation 13(1):83
Hou T-T, Yang H-Y, Wang W, Wu Q-Q, Tian Y-R, Jia J-P (2018) Sulforaphane inhibits the generation of amyloid-β oligomer and promotes spatial learning and memory in Alzheimer’s disease (PS1V97L) transgenic mice. J Alzheimers Dis (preprint) 1–11
Hsu A, Wong CP, Yu Z, Williams DE, Dashwood RH, Ho E (2011) Promoter de-methylation of cyclin D2 by sulforaphane in prostate cancer cells. Clin Epigenetics 3(1):3
Hu R, Hebbar V, Kim B-R, Chen C, Winnik B, Buckley B, Soteropoulos P, Tolias P, Hart RP, Kong A-NT (2004) In vivo pharmacokinetics and regulation of gene expression profiles by isothiocyanate sulforaphane in the rat. J Pharmacol Exp Ther 310(1):263–271
Hu C, Eggler AL, Mesecar AD, Van Breemen RB (2011) Modification of keap1 cysteine residues by sulforaphane. Chem Res Toxicol 24(4):515–521
Hullar MA, Fu BC (2014) Diet, the gut microbiome, and epigenetics. Cancer J (Sudbury, Mass.) 20(3):170
Hwang JS, Hwang JS, Chang I, Kim S (2007) Age-associated decrease in proteasome content and activities in human dermal fibroblasts: restoration of normal level of proteasome subunits reduces aging markers in fibroblasts from elderly persons. J Gerontol Ser A Biol Med Sci 62(5):490–499
Iizumi T, Takahashi S, Mashima K, Minami K, Izawa Y, Abe T, Hishiki T, Suematsu M, Kajimura M, Suzuki N (2016) A possible role of microglia-derived nitric oxide by lipopolysaccharide in activation of astroglial pentose-phosphate pathway via the Keap1/Nrf2 system. J Neuroinflammation 13(1):99
Ishida M, Hara M, Fukino N, Kakizaki T, Morimitsu Y (2014) Glucosinolate metabolism, functionality and breeding for the improvement of Brassicaceae vegetables. Breed Sci 64(1):48–59
Jang M, Cho I-H (2016) Sulforaphane ameliorates 3-nitropropionic acid-induced striatal toxicity by activating the Keap1-Nrf2-ARE pathway and inhibiting the MAPKs and NF-κB pathways. Mol Neurobiol 53(4):2619–2635
Jazwa A, Rojo AI, Innamorato NG, Hesse M, Fernández-Ruiz J, Cuadrado A (2011) Pharmacological targeting of the transcription factor Nrf2 at the basal ganglia provides disease modifying therapy for experimental parkinsonism. Antioxid Redox Signal 14(12):2347–2360
Jhang KA, Park J-S, Kim H-S, Chong YH (2018) Sulforaphane rescues amyloid-β peptide-mediated decrease in MerTK expression through its anti-inflammatory effect in human THP-1 macrophages. J Neuroinflammation 15(1):75
Jones DP (2015) Redox theory of aging. Redox Biol 5:71–79
Kahn NW, Rea SL, Moyle S, Kell A, Johnson TE (2008) Proteasomal dysfunction activates the transcription factor SKN-1 and produces a selective oxidative-stress response in Caenorhabditis elegans. Biochem J 409(1):205–213
Kapeta S, Chondrogianni N, Gonos ES (2010) Nuclear erythroid factor 2 (Nrf2) mediated proteasome activation delays senescence in human fibroblasts. J Biol Chem 285(11):8171–8184
Katsiki M, Chondrogianni N, Chinou I, Rivett AJ, Gonos ES (2007) The olive constituent oleuropein exhibits proteasome stimulatory properties in vitro and confers life span extension of human embryonic fibroblasts. Rejuvenation Res 10(2):157–172
Kim J, Lee S, Choi BR, Yang H, Hwang Y, Park JHY, LaFerla FM, Han JS, Lee KW, Kim J (2017) Sulforaphane epigenetically enhances neuronal BDNF expression and TrkB signaling pathways. Mol Nutr Food Res 61(2):1600194
Kubo E, Chhunchha B, Singh P, Sasaki H, Singh DP (2017) Sulforaphane reactivates cellular antioxidant defense by inducing Nrf2/ARE/Prdx6 activity during aging and oxidative stress. Sci Rep 7(1):14130
Kwak M-K, Wakabayashi N, Greenlaw JL, Yamamoto M, Kensler TW (2003) Antioxidants enhance mammalian proteasome expression through the Keap1-Nrf2 signaling pathway. Mol Cell Biol 23(23):8786–8794
Kwak M-K, Cho J-M, Huang B, Shin S, Kensler TW (2007) Role of increased expression of the proteasome in the protective effects of sulforaphane against hydrogen peroxide-mediated cytotoxicity in murine neuroblastoma cells. Free Radic Biol Med 43(5):809–817
Lai R-H, Miller MJ, Jeffery E (2010) Glucoraphanin hydrolysis by microbiota in the rat cecum results in sulforaphane absorption. Food Funct 1(2):161–166
Lawrence T (2009) The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb Perspect Biol a001651
Li J, Johnson D, Calkins M, Wright L, Svendsen C, Johnson J (2004) Stabilization of Nrf2 by tBHQ confers protection against oxidative stress-induced cell death in human neural stem cells. Toxicol Sci 83(2):313–328
Li B, Cui W, Liu J, Li R, Liu Q, Xie X-H et al (2013) Sulforaphane ameliorates the development of experimental autoimmune encephalomyelitis by antagonizing oxidative stress and Th17-related inflammation in mice. Exp Neurol 250:239–249
Li Y, Buckhaults P, Cui X, Tollefsbol TO (2016) Combinatorial epigenetic mechanisms and efficacy of early breast cancer inhibition by nutritive botanicals. Epigenomics 8(8):1019–1037
Liddell J (2017) Are astrocytes the predominant cell type for activation of Nrf2 in aging and neurodegeneration? Antioxidants 6(3):65
Liu Y, Liu X, Zhang T, Luna C, Liton PB, Gonzalez P (2007) Cytoprotective effects of proteasome β5 subunit overexpression in lens epithelial cells. Mol Vis 13:31
Liu Y, Hettinger CL, Zhang D, Rezvani K, Wang X, Wang H (2014) Sulforaphane enhances proteasomal and autophagic activities in mice and is a potential therapeutic reagent for Huntington's disease. J Neurochem 129(3):539–547
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153(6):1194–1217
Martinez-Villaluenga C, Peñas E, Ciska E, Piskula MK, Kozlowska H, Vidal-Valverde C, Frias J (2010) Time dependence of bioactive compounds and antioxidant capacity during germination of different cultivars of broccoli and radish seeds. Food Chem 120(3):710–716
Meeran SM, Patel SN, Tollefsbol TO (2010) Sulforaphane causes epigenetic repression of hTERT expression in human breast cancer cell lines. PLoS One 5(7):e11457
Morroni F, Tarozzi A, Sita G, Bolondi C, Moraga JMZ, Cantelli-Forti G, Hrelia P (2013) Neuroprotective effect of sulforaphane in 6-hydroxydopamine-lesioned mouse model of Parkinson's disease. Neurotoxicology 36:63–71
Myzak MC, Karplus PA, Chung F-L, Dashwood RH (2004) A novel mechanism of chemoprotection by sulforaphane: inhibition of histone deacetylase. Cancer Res 64(16):5767–5774
Myzak MC, Dashwood WM, Orner GA, Ho E, Dashwood RH (2006a) Sulforaphane inhibits histone deacetylase in vivo and suppresses tumorigenesis in Apc min mice. FASEB J 20(3):506–508
Myzak MC, Ho E, Dashwood RH (2006b) Dietary agents as histone deacetylase inhibitors. Mol Carcinog 45(6):443–446
Nallasamy P, Si H, Babu PVA, Pan D, Fu Y, Brooke EA et al (2014) Sulforaphane reduces vascular inflammation in mice and prevents TNF-α-induced monocyte adhesion to primary endothelial cells through interfering with the NF-κB pathway. J Nutr Biochem 25(8):824–833
Negi G, Kumar A, Sharma S, S. (2011) Nrf2 and NF-κB modulation by sulforaphane counteracts multiple manifestations of diabetic neuropathy in rats and high glucose-induced changes. Curr Neurovasc Res 8(4):294–304
Oeckinghaus A, Ghosh S (2009) The NF-κB family of transcription factors and its regulation. Cold Spring Harbor Perspect Biol a000034
Organization WH (2015) World report on ageing and health. World Health Organization
Papaevgeniou N, Chondrogianni N (2016) UPS activation in the battle against aging and aggregation-related diseases: an extended review. In: Proteostasis. Springer, pp 1–70
Papaevgeniou N, Sakellari M, Jha S, Tavernarakis N, Holmberg CI, Gonos ES, Chondrogianni N (2016) 18α-Glycyrrhetinic acid proteasome activator decelerates aging and Alzheimer’s disease progression in Caenorhabditis elegans and neuronal cultures. Antioxid Redox Signal 25(16):855–869
Park H-M, Kim J-A, Kwak M-K (2009) Protection against amyloid beta cytotoxicity by sulforaphane: role of the proteasome. Arch Pharm Res 32(1):109–115
Paul B, Li Y, Tollefsbol T (2018) The effects of combinatorial genistein and sulforaphane in breast tumor inhibition: role in epigenetic regulation. Int J Mol Sci 19(6):1754
Pennisi M, Crupi R, Di Paola R, Ontario ML, Bella R, Calabrese EJ, … Calabrese V (2017) Inflammasomes, hormesis, and antioxidants in neuroinflammation: role of NRLP3 in Alzheimer disease. J Neurosci Res 95(7):1360–1372
Petri N, Tannergren C, Holst B, Mellon FA, Bao Y, Plumb GW, … Knutson L (2003) Absorption/metabolism of sulforaphane and quercetin, and regulation of phase II enzymes, in human jejunum in vivo. Drug Metab Dispos 31(6):805–813
Pickering AM, Linder RA, Zhang H, Forman HJ, Davies KJ (2012) Nrf2-dependent induction of proteasome and Pa28αβ regulator are required for adaptation to oxidative stress. J Biol Chem 287(13):10021–10031
Pontius AT, Smith PW (2011) An antiaging and regenerative medicine approach to optimal skin health. Facial Plast Surg 27(01):029–034
Poprac P, Jomova K, Simunkova M, Kollar V, Rhodes CJ, Valko M (2017) Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol Sci 38(7):592–607
Prestera T, Zhang Y, Spencer SR, Wilczak CA, Talalay P (1993) The electrophile counterattack response: protection against neoplasia and toxicity. Adv Enzym Regul 33:281–296
Prochaska HJ, Santamaria AB, Talalay P (1992) Rapid detection of inducers of enzymes that protect against carcinogens. Proc Natl Acad Sci 89(6):2394–2398
Pu D, Zhao Y, Chen J, Lv A, Zhu S, Luo C, … Xiao Q (2018) Protective effects of sulforaphane on cognitive impairments and AD-like lesions in diabetic mice are associated with the upregulation of Nrf2 transcription activity. Neuroscience 381:35–45
Royston KJ, Udayakumar N, Lewis K, Tollefsbol TO (2017) A novel combination of withaferin A and sulforaphane inhibits epigenetic machinery, cellular viability and induces apoptosis of breast cancer cells. Int J Mol Sci 18(5):1092
Sachdeva MM, Cano M, Handa JT (2014) Nrf2 signaling is impaired in the aging RPE given an oxidative insult. Exp Eye Res 119:111–114
Sharpless NE, DePinho RA (2007) How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol 8(9):703–713
Shih VF-S, Tsui R, Caldwell A, Hoffmann A (2011) A single NFκB system for both canonical and non-canonical signaling. Cell Res 21(1):86–102
Shirai Y, Fujita Y, Hashimoto R, Ohi K, Yamamori H, Yasuda Y, … Takeda M (2015) Dietary intake of sulforaphane-rich broccoli sprout extracts during juvenile and adolescence can prevent phencyclidine-induced cognitive deficits at adulthood. PLoS One. 10(6):e0127244
Silva-Palacios A, Ostolga-Chavarria M, Zazueta C, Königsberg M (2018) Nrf2: molecular and epigenetic regulation during aging. Ageing Res Rev 47:31–40
Sun S-C (2017) The non-canonical NF-κB pathway in immunity and inflammation. Nat Rev Immunol 17(9):545–558
Sunkaria A, Bhardwaj S, Yadav A, Halder A, Sandhir R (2018) Sulforaphane attenuates postnatal proteasome inhibition and improves spatial learning in adult mice. J Nutr Biochem 51:69–79
Talalay P, De Long MJ, Prochaska HJ (1988) Identification of a common chemical signal regulating the induction of enzymes that protect against chemical carcinogenesis. Proc Natl Acad Sci 85(21):8261–8265
Talalay P, Fahey JW, Holtzclaw WD, Prestera T, Zhang Y (1995) Chemoprotection against cancer by phase 2 enzyme induction. Toxicol Lett 82:173–179
Talalay P, Fahey JW, Healy ZR, Wehage SL, Benedict AL, Min C, Dinkova-Kostova AT (2007) Sulforaphane mobilizes cellular defenses that protect skin against damage by UV radiation. Proc Natl Acad Sci 104(44):17500–17505
Tonoki A, Kuranaga E, Tomioka T, Hamazaki J, Murata S, Tanaka K, Miura M (2009) Genetic evidence linking age-dependent attenuation of the 26S proteasome with the aging process. Mol Cell Biol 29(4):1095–1106
Tortorella SM, Royce SG, Licciardi PV, Karagiannis TC (2015) Dietary sulforaphane in cancer chemoprevention: the role of epigenetic regulation and HDAC inhibition. Antioxid Redox Signal 22(16):1382–1424
Townsend BE, Johnson RW (2016) Sulforaphane induces Nrf2 target genes and attenuates inflammatory gene expression in microglia from brain of young adult and aged mice. Exp Gerontol 73:42–48
Verkerk R, Schreiner M, Krumbein A, Ciska E, Holst B, Rowland I, … Mithen R (2009) Glucosinolates in Brassica vegetables: the influence of the food supply chain on intake, bioavailability and human health. Mol Nutr Food Res 53(S2):S219–S219
Vilchez D, Morantte I, Liu Z, Douglas PM, Merkwirth C, Rodrigues AP, … Dillin A (2012) RPN-6 determines C. elegans longevity under proteotoxic stress conditions. Nature 489(7415):263
Vilchez D, Saez I, Dillin A (2014) The role of protein clearance mechanisms in organismal ageing and age-related diseases. Nat Commun 5:5659
Winkler S, Faragher J, Franz P, Imsic M, Jones R (2007) Glucoraphanin and flavonoid levels remain stable during simulated transport and marketing of broccoli (Brassica oleracea var. italica) heads. Postharvest Biol Technol 43(1):89–94
Xu C, Li CY-T, Kong A-NT (2005) Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch Pharm Res 28(3):249–268
Yang H, Liu F, Li Y, Yu B (2017) Reconstructing biosynthetic pathway of the plant-derived cancer chemopreventive-precursor glucoraphanin in Escherichia coli. ACS Synth Biol 7(1):121–131
Zanichelli F, Capasso S, Cipollaro M, Pagnotta E, Cartenì M, Casale F, Iori R, Galderisi U (2012) Dose-dependent effects of R-sulforaphane isothiocyanate on the biology of human mesenchymal stem cells, at dietary amounts, it promotes cell proliferation and reduces senescence and apoptosis, while at anti-cancer drug doses, it has a cytotoxic effect. Age 34(2):281–293
Zhang Y, Talalay P, Cho CG, Posner GH (1992) A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci U S A 89(6):2399–2403
Zhang H, Davies KJ, Forman HJ (2015) Oxidative stress response and Nrf2 signaling in aging. Free Radic Biol Med 88:314–336
Zhang J, Zhang R, Zhan Z, Li X, Zhou F, Xing A, Jiang C, Chen Y, An L (2017) Beneficial effects of sulforaphane treatment in Alzheimer’s disease may be mediated through reduced HDAC1/3 and increased P75NTR expression. Front Aging Neurosci 9:121
Zhao Z, Liao G, Zhou Q, Lv D, Holthfer H, Zou H (2016) Sulforaphane attenuates contrast-induced nephropathy in rats via Nrf2/HO-1 pathway. Oxidative Med Cell Longev
Zhao F, Zhang J, Chang N (2018) Epigenetic modification of Nrf2 by sulforaphane increases the antioxidative and anti-inflammatory capacity in a cellular model of Alzheimer’s disease. Eur J Pharmacol 824:1–10
Zhou Q, Chen B, Wang X, Wu L, Yang Y, Cheng X, … Sun X (2016) Sulforaphane protects against rotenone-induced neurotoxicity in vivo: involvement of the mTOR, Nrf2, and autophagy pathways. Sci Rep 6:32206
Zhou L, Zhang H, Davies KJ, Forman HJ (2018) Aging-related decline in the induction of Nrf2-regulated antioxidant genes in human bronchial epithelial cells. Redox Biol 14:35–40
Zhu M, Zhang Y, Cooper S, Sikorski E, Rohwer J, Bowden GT (2004) Phase II enzyme inducer, sulforaphane, inhibits UVB-induced AP-1 activation in human keratinocytes by a novel mechanism. Mol Carcinog 41(3):179–186
Funding
This work was supported by CONACyT grant FON.INST/298/2016, as well as the “Red Temática de Investigación en Salud y Desarrollo Social” from CONACYT, Mexico. Santín-Márquez is a CONACyT scholarship holder. Research from NC lab is currently co-financed by the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, (a) under the call RESEARCH – CREATE – INNOVATE (project code: T1EDK-00353 and T1EDK-01610) and (b) under the Action “Action for the Strategic Development on the Research and Technological Sector” (project STHENOS-b, MIS 5002398).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Santín-Márquez, R., Alarcón-Aguilar, A., López-Diazguerrero, N.E. et al. Sulforaphane - role in aging and neurodegeneration. GeroScience 41, 655–670 (2019). https://doi.org/10.1007/s11357-019-00061-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-019-00061-7