Abstract
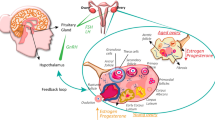
Cardiovascular disease, rare in premenopausal women, increases sharply at menopause and is typically accompanied by chronic inflammation. Previous work in our laboratory demonstrated that replacing senescent ovaries in post-reproductive mice with young, actively cycling ovaries restored many health benefits, including decreased cardiomyopathy and restoration of immune function. Our objective here was to determine if depletion of germ cells from young transplanted ovaries would alter the ovarian-dependent extension of life and health span. Sixty-day-old germ cell-depleted and germ cell-containing ovaries were transplanted to post-reproductive, 17-month-old mice. Mean life span for female CBA/J mice is approximately 644 days. Mice that received germ cell-containing ovaries lived 798 days (maximum = 815 days). Mice that received germ cell-depleted ovaries lived 880 days (maximum = 1046 days), 29% further past the time of surgery than mice that received germ cell-containing ovaries. The severity of inflammation was reduced in all mice that received young ovaries, whether germ cell-containing or germ cell-depleted. Aging-associated inflammatory cytokine changes were reversed in post-reproductive mice by 4 months of new-ovary exposure. In summary, germ cell depletion enhanced the longevity-extending effects of the young, transplanted ovaries and, as with germ cell-containing ovaries, decreased the severity of inflammation, but did so independent of germ cells. Based on these observations, we propose that gonadal somatic cells are programed to preserve the somatic health of the organism with the intent of facilitating future germline transmission. As reproductive potential decreases or is lost, the incentive to preserve the somatic health of the organism is lost as well.








Similar content being viewed by others
References
Arantes-Oliveira N, Apfeld J, Dillin A, Kenyon C (2002) Regulation of life-span by germ-line stem cells in Caenorhabditis elegans. Science 295(5554):502–505
Ashpole NM, Logan S, Yabluchanskiy A, Mitschelen MC, Yan H, Farley JA, Hodges E, LUngvari Z, Csiszar A, Chen S, Georgescu C, Hubbard GB, Ikeno Y, Sonntag WE (2017) IGF-1 has sexually dimorphic, pleiotropic, and time-dependent effects on healthspan, pathology, and lifespan. GeroScience 39(2):129–145
Barnett KR, Schilling C, Greenfeld CR, Tomic D, Flaw JA (2006) Ovarian follicle development and transgenic mouse models. Hum Reprod Update 12(5):537–555. https://doi.org/10.1093/humupd/dml022
Bronson RT, Lipman RD (1991) Reduction in rate of occurrence of age-related lesions in dietary restricted laboratory mice. Growth Dev Aging 55(3):169–184
Cargill SL, Medrano JF, Anderson GB (1999) Infertility in a line of mice with the high growth mutation is due to luteal insufficiency resulting from disruption at the hypothalamic-pituitary axis. Biol Reprod 61(1):283–287
Cargill SL, Carey JR, Muller HG, Anderson GB (2003) Age of ovary determines remaining life expectancy in old ovariectomized mice. Aging Cell 2(3):185–190
Carrero J, Stenvinkel P (2009) Persistent inflammation as a catalyst for other risk factors in chronic kidney disease: a hypothesis proposal. Clin J Am Soc Nephrol 4(Suppl 1):S49–S55. https://doi.org/10.2215/CJN.02720409
Casalino SM, Linares JA, Goldraij A (1994) Different effect of a restricted diet on isolated uteri of ovariectomized and non-ovariectomized rats. Influence of indomethacin and prostaglandins. Prostaglandins Leukot Essent Fat Acids 51(1):41–45. https://doi.org/10.1016/0952-3278(94)90176-7
Deepa SS, Bhaskaran S, Espinoza S, Brooks SV, McArdle A, Jackson MJ, Van Remmen H, Richardson A (2017) A new mouse model of frailty: the Cu/Zn superoxide dismutase knockout mouse. GeroScience 39(2):187–198
Eisenberg ML, Li SF, Behr B, Cullen MR, Galusha D, Lamb DJ, Lipshultz LI (2014) Semen quality, infertility and mortality in the USA. Hum Reprod 29(7):1567–1574. https://doi.org/10.1093/humrep/deu106
Faddy MJ, Telfer E, Gosden RG (1987) The kinetics of pre-antral follicle development in ovaries of CBA/Ca mice during the first 14 weeks of life. Cell Tissue Kinet 20:551–560
Flatt T, Min K, D’Alterio C, Villa-Cuesta E, Cumbers J, Lehmann R, Jones D, Tatar M (2008) Drosophila germ-line modulation of insulin signaling and lifespan. Proc Natl Acad Sci U S A 105:6368–6373. https://doi.org/10.1073/pnas
Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G (2000) Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 908:244–254. https://doi.org/10.1111/j.1749-6632.2000.tb06651
Frasca D, Blomberg BB (2016) Inflammaging decreases adaptive and innate immune responses in mice and humans. Biogerontology 17(1):7–19. https://doi.org/10.1007/s10522-015-9578-8
Gosden RG, Jones EC, Jacks F (1978) Pituitary-ovarian relationships during post-reproductive phase of inbred mice. Exp Gerontol 13:159–166. https://doi.org/10.1016/0531-5565(78)90008-6
Gregor MF, Hotamisligil GS (2011) Inflammatory mechanisms in obesity. Annu Rev Immunol 29:415–445. https://doi.org/10.1146/annurev-immunol-031210-101322
Hsin H, Kenyon C (1999) Signals from the reproductive system regulate the lifespan of C-elegans. Nature 399:362–366. https://doi.org/10.1038/20694
Hughes GC, Clark EA (2007) Regulation of dendritic cells by female sex steroids: relevance to immunity and autoimmunity. Autoimmunity 40:470–481. https://doi.org/10.1080/08916930701464764
Hughes GC, Martin D, Zhang K, Hudkins HL, Alpers CL, Clark EA, Elkon KB (2009) Decrease in glomerulonephritis and Th1-associated autoantibody production after progesterone treatment in NZB/NZW mice. Arthritis Rheum 60:1775–1784. https://doi.org/10.1002/art.24548
Ikeno Y, Hubbard GB, Lee S, Richardson A, Strong R, Diaz V, Nelson JF (2005) Housing density does not influence the longevity effect of calorie restriction. J Gerontol A Biol Sci Med Sci 60(12):1510–1517
Jacobsen BK, Knutsen SF, Fraser GE (1999) Age at natural menopause and total mortality and mortality from ischemic heart disease: the Adventist health study. J Clin Epidemiol 52(4):303–307
Johnell O, Kanis JA (2006) An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17(12):1726–1733. https://doi.org/10.1007/s00198-006-0172-4
Jones EC, Krohn PL (1961) Relationships between age, numbers of oocytes and fertility in virgin and multiparious mice. J Endocrinol 21:469–495. https://doi.org/10.1677/joe.0.0210469
Kulaksizoglu M, Ipekci SH, Kebapcilar L, Kebapcilar AG, Korkmaz H, Akyurek F, Baldane S, Gonen MS (2013) Risk factors for diabetes mellitus in women with primary ovarian insufficiency. Biol Trace Elem Res 154(3):313–320. https://doi.org/10.1007/s12011-013-9738-0
Longo VD, Finch CE (2003) Evolutionary medicine: from dwarf model systems to healthy centenarians? Science 299(5611):1342–1346. https://doi.org/10.1126/science.1077991
Marriott LK, Hauss-Wegrzyniak B, Benton RS, Vraniak PD, Wenk GL (2002) Long-term estrogen therapy worsens the behavioral and neuropathological consequences of chronic brain inflammation. Behav Neurosci 116(5):902–911. https://doi.org/10.1037//0735-7044.116.5.902
Mason JC, Libby P (2014) Cardiovascular disease in patients with chronic inflammation: mechanisms underlying premature cardiovascular events in rheumatologic conditions. Eur Heart J 36:482–489. https://doi.org/10.1093/eurheartj/ehu403
Mason JB, Cargill SL, Anderson GB, Carey JR (2009) Transplantation of young ovaries to old mice increased life span in transplant recipients. J Gerontol A Biol Sci Med Sci 64(12):1207–1211. https://doi.org/10.1093/gerona/glp134
Mason JB, Cargill SL, Anderson GB, Carey JR (2010) Ovarian status influenced the rate of body-weight change but not the total amount of body-weight gained or lost in female CBA/J mice. Exp Gerontol 45:435–441. https://doi.org/10.1016/j.exger.2010.03.010
Mason JB, Cargill SL, Griffey SM, Reader JR, Anderson GB, Carey JR (2011) Transplantation of young ovaries restored cardioprotective influence in post-reproductive-aged mice. Aging Cell 10:448–456. https://doi.org/10.1111/j.1474-9726.2011.00691.x
Mason JB, Terry BC, Merchant SS, Mason HM, Nazokkarmaher M (2015) Manipulation of ovarian function significantly influenced trabecular and cortical bone volume, architecture and density in mice at death. PLoS One 10(12):e0145821. https://doi.org/10.1371/journal.pone.0145821
Mason JB, Parkinson KC, Habermehl TL (2018) Orthotopic ovarian transplantation procedures to investigate the life- and health-span influence of ovarian senescence in female mice. J Vis Exp (132):e56638. https://doi.org/10.3791/56638
Monteiro R, Teixeira D, Calhau C (2014) Estrogen signaling in metabolic inflammation. Mediat Inflamm 615917:1–20. https://doi.org/10.1155/2014/615917
National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Fertility and Infertility Branch. New research priorities. 'Fertility status as a marker of overall health'. 2016. "Support studies that investigate fertility status as a marker of overall health for both men and women"
Nelson JF, Gosden RG, Felicio LS (1985) Effect of dietary restriction on estrous cyclicity and follicular reserves in aging C57/BL6 mice. Biol Reprod 32(3):515–522. https://doi.org/10.1095/biolreprod32.3.515
Panda A, Arjona A, Sapey E, Bai F, Fikrig E, Montgomery RR, Lord JM, Shaw AC (2009) Human innate immunosenescence: causes and consequences for immunity in old age. Trends Immunol 30(7):325–333. https://doi.org/10.1016/j.it.2009.05.004
Parkinson KC, Peterson RL, Mason JB (2017) Cognitive behavior and sensory function were significantly influenced by restoration of active ovarian function in post-reproductive mice. Exp Gerontol 92:28–33. https://doi.org/10.1016/j.exger.2017.03.002
Peterson RL, Parkinson KC, Mason JB (2016) Manipulation of ovarian function significantly influenced sarcopenia in post-reproductive-age mice. J Transplant. https://doi.org/10.1155/2016/4570842
Peterson RL, Parkinson KC, Mason JB (2017) Immune and renal function, which are critical for reproductive success suffer substantial declines in aged females, but are significantly restored by re-establishment of active ovarian function in post-reproductive females. Reprod Fertil Dev. https://doi.org/10.1071/RD16333
Podlutsky A, Valcarcel-Ares MN, Yancey K, Podlutskaya V, Nagykaldi E, Gautam T, Miller RL, Sonntag WE, Csiszár A, Ungvari Z (2017) The GH/IGF-1 axis in a critical period early in life determines cellular DNA repair capacity by altering transcriptional regulation of DNA repair-related genes: implications for the developmental origins of cancer. GeroScience 39:147–160
Rais M, Wilson RM, Urbanski HF, Messaoudi I (2017) Androgen supplementation improves some but not all aspects of immune senescence in aged male macaques. GeroScience 39:373–384
Rivera Z, Christian PJ, Marion SL, Brooks HL, Hoyer PB. Steroidogenic capacity of residual ovarian tissue in 4-vinylcyclohexene diepoxide-treated mice. Biol. Reprod. 2009;80:328–336
Rosario ER, Chang L, Head EH, Stanczyk FZ, Pike CJ (2011) Brain levels of sex steroid hormones in men and women during normal aging and in Alzheimer’s disease. Neurobiol Aging 32(4):604–613. https://doi.org/10.1016/j.neurobiolaging.2009.04.008
Selesniemi K, Lee HJ, Tilly JL (2008) Moderate caloric restriction initiated in rodents during adulthood sustains function of the female reproductive axis into advanced chronological age. Aging Cell 7(5):622–629. https://doi.org/10.1111/j.1474-9726.2008.00409.x
Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA (2010) Premature menopause or early menopause: Long-term health consequences. Maturitas 65(2):161–166. https://doi.org/10.1016/j.maturitas.2009
Sparkman NL, Johnson RW (2008) Neuroinflammation associated with aging sensitizes the brain to the effects of infection or stress. Neuroimmunomodulation 15(4–6):323–330. https://doi.org/10.1159/000156474
Sterin AB, Linares JA, Goldraij A (1989) Effect of dietary restriction on triglyceride levels in the uterus isolated from pregnant rats. Influences of prostaglandins and indomethacin. Prostaglandins Leukot Essent Fatty Acids 38(2):129–135. https://doi.org/10.1677/joe.0.1710463.
Stork S, Vonschacky C, Angerer P (2002) The effect of 17 beta-estradiol on endothelial and inflammatory markers in post-menopausal women: a randomized and controlled trial. Atherosclerosis 165:301–307
Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O'Donnell C, Kittner S, Lloyd-Jones D, Goff DC Jr, Hong Y, Adams R, Friday G, Furie K, Gorelick P, Kissela B, Marler J, Meigs J, Roger V, Sidney S, Sorlie P, Steinberger J, Wasserthiel-Smoller S, Wilson M, Wolf P, American Heart Association Statistics Committee and Stroke Statistics Subcommittee (2006) Heart disease and stroke statistics—2006 update—a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 113(6):E85–E151. https://doi.org/10.1161/circulationaha.105.171600
Thung PJ, Boot LM, Muhlbock O (1956) Senile changes in the oestrous cycle and in ovarian structure in some inbred strains of mice. Acta Endocrinol 23:8–32. https://doi.org/10.1530/acta.0.0230008
Ungvari Z, Valcarcel-Ares MN, Tarantini S, Yabluchanskiy A, Fülöp GÁ, Kiss T, Csiszár A (2017) Connective tissue growth factor (CTGF) in age-related vascular pathologies. GeroScience 39:491–498
Van Diepen JA, Berbée JF, Havekes LM, Rensen PC (2013) Interactions between inflammation and lipid metabolism: relevance for efficacy of anti-inflammatory drugs in the treatment of atherosclerosis. Atherosclerosis 228(2):306–315. https://doi.org/10.1016/j.atherosclerosis.2013.02.028
Xie Z, Morgan TE, Rozovsky I, Finch CE (2003) Aging and glial responses to lipopolysaccharide in vitro: greater induction of IL-1 and IL-6, but smaller induction of neurotoxicity. Exp Neurol 182(1):135–141
Yoshida T, Takahashi K, Yamatani H, Takata K, Kurachi H (2011) Impact of surgical menopause on lipid and bone metabolism. Climacteric 14:445–452. https://doi.org/10.3109/13697137.2011.562994
Yuan R, Tsaih SW, Petkova SB, Marin de Evsikova C, Xing S, Marion MA, Bogue MA, Mills KD, Peters LL, Bult CJ, Rosen CJ, Sundberg JP, Harrison DE, Churchill GA, Paigen B (2009) Aging in inbred strains of mice: study design and interim report on median lifespans and circulating IGF1 levels. Aging Cell 8(3):277–287. https://doi.org/10.1111/j.1474-9726.2009.00478.x
Acknowledgments
The authors thank Dr. Aaron Olsen and Ms. Lisa DeSoi for help with the mice, Dr. Erik Eide and Mr. Mahdi Nazokkarmaher for help with VCD injections, and Ms. Sumira Phatak for guidance with magnetic resonance imaging. The authors also thank Dr. Shelley Cargill and Dr. Chris Pearl for critical review of the manuscript. Research reported in this publication was supported by Utah State University, School of Veterinary Medicine, Department of Animal, Dairy and Veterinary Sciences Research Initiation Funds, the Cluster of Excellence for Aging Research (CECAD) at the University of Cologne and by a generous gift of aged CBA/J female mice from Nancy Nadon at the National Institute on Aging.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: TH, JM. Performed the experiments: TH, KP, GH, YI, JE, BS, JM. Analyzed the data: TH, GH, YI, JE, BS, JM. Contributed reagents/materials/analysis tools: YI, BS, JM. Wrote the paper: TH, KP, JM.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Habermehl, T.L., Parkinson, K.C., Hubbard, G.B. et al. Extension of longevity and reduction of inflammation is ovarian-dependent, but germ cell-independent in post-reproductive female mice. GeroScience 41, 25–38 (2019). https://doi.org/10.1007/s11357-018-0049-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-018-0049-4