Abstract
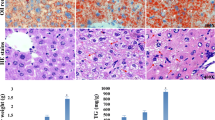
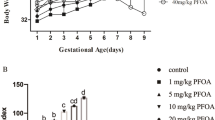
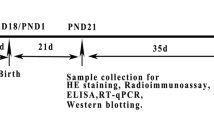
The study was conducted to investigate the liver toxicity in female offspring mice induced by maternal exposure to perfluorooctanoic acid (PFOA). Fifty pregnant Kunming mice were randomly divided into 5 groups with 10 of each, which were treated with 0.2 mL PFOA solution dissolved with deionized water at 0, 1, 2.5, 5, and 10 mg/kg BW, respectively, from the pregnancy day (PND) 0 to day 17. Female offspring mice were sacrificed to collect serum and liver at postpartum day 21. The results showed that PFOA significantly reduced the body weight at weaning and the survival rate of the female offspring mice (P < 0.01) increased the liver index of the pups (P < 0.01). Meanwhile, PFOA also caused hepatic bleeding, local necrosis, and enlargement of hepatocytes and vacuolization. The levels of serum AST, ALT, SOD, and CAT in PFOA treatment group were upregulated significantly (P < 0.01). The expressions of Acot1, Acox1, and Acsl1 genes were increased significantly (P < 0.01). The expression of PPAR-α gene was decreased significantly (P < 0.01). There was no significant difference in the expression of Cpt1a gene among the 5 groups. HAT activity was reduced significantly and HDAC activity was increased significantly. The expression of anti-acetyl-histone H3 and acetyl-histone H4 was reduced significantly. Thus, our findings indicate that exposure to PFOA during pregnancy affects the growth and development of the pups and causes liver damage, disrupting the secretion of enzymes involved in fatty acid oxidation induced by PPAR-α, leading to liver oxidative stress and a decrease in the degree of histone acetylation. Elevated HDAC may aggravate downstream fatty acid metabolism disorders through PPAR-α.




Similar content being viewed by others
References
Abbott BD, Wood CR, Watkins AM, Tatum-Gibbs K, Das KP, Lau C (2012) Effects of perfluorooctanoic acid (PFOA) on expression of peroxisome proliferator-activated receptors (PPAR) and nuclear receptor-regulated genes in fetal and postnatal CD-1 mouse tissues. Reprod Toxicol 33(4):491–505
Azhar M, Schultz JEJ, Grupp I, Dorn GW 2nd, Meneton P, Molin DG, Gittenberger-de Groot AC, Doetschman T (2003) Transforming growth factor beta, in cardiovascular development and function. Cytokine Growth Factor Rev 14(5):391–407
Berthiaume M, Boufaied N, Moisan A, Gaudreau L (2006) High levels of oxidative stress globally inhibit gene transcription and histone acetylation. DNA Cell Biol 25(2):124–134
Buhrke T, Krüger E, Pevny S, Rößler M, Bitter K, Lampen A (2015) Perfluorooctanoic acid (PFOA) affects distinct molecular signalling pathways in human primary hepatocytes. Toxicology 333:53–62
Chen T, Zhang L, Yue JQ, Lv ZQ, Xia W, Wan YJ, Li YY, Xu SQ (2012) Prenatal PFOS exposure induces oxidative stress and apoptosis in the lung of rat off-spring. Reprod Toxicol 33(4):538–545
Cui Q, Pan Y, Zhang H, Sheng N, Dai J (2018) Elevated concentrations of perfluorohexanesulfonate and other per- and polyfluoroalkyl substances in Baiyangdian Lake (China): Source characterization and exposure assessment. Environ Pollut 241:684–691
Das KP, Wood CR, Lin MT, Starkov AA, Lau C, Wallace KB, Corton JC, Abbott BD (2017) Perfluoroalkyl acids-induced liver steatosis: effects on genes controlling lipid homeostasis. Toxicology 378:37–52
Dekker FJ, Bosch TVD, Martin NI (2014) Small molecule inhibitors of histone acetyltransferases and deacetylases are potential drugs for inflammatory diseases. Drug Discov Today 19(5):654–660
Ellis JM, Li LO, Wu PC (2010) Adipose Acyl-CoA Synthetase-1 Directs fatty acids toward β-oxidation and is required for cold thermogenesis. Cell Metab 12(1):53–64
Falkenberg KJ, Johnstone RW (2014) Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nat Rev Drug Discov 13(9):673–691
Haberland M, Montgomery RL, Olson EN (2009) The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet 10(1):32–42
Hideshima T, Anderson KC (2013) Histone deacetylase inhibitors in the treatment for multiple myeloma. Int J Hematol 97(3):324–332
Huang Q, Zhang J, Martin FL (2013) Perfluorooctanoic acid induces apoptosis through the p53-dependent mitochondrial pathway in human hepatic cells: a proteomic study. Toxicol Lett 223(2):211–220
Hui Z, Li R, Chen L (2017) The impact of exposure to environmental contaminant on hepatocellular lipid metabolism. Gene 622:67–71
Keppler BR, Archer TK (2008a) Chromatin-modifying enzymes as therapeutic targets--Part 1. Expert Opin Ther Targets 12(10):1301–1312
Keppler BR, Archer TK (2008b) Chromatin-modifying enzymes as therapeutic targets--Part 2. Expert Opin Ther Targets 12(11):1457–1467
Khan SA, Sathyanarayan A, Mashek MT, Ong KT, Wollaston-Hayden EE, Mashek DG (2015) ATGL-catalyzed lipolysis regulates SIRT1 to control PGC-1α/PPAR-α signaling. Diabetes 64(2):418–426
Lau C, Thibodeaux JR, Hanson RG, Narotsky MG, Rogers JM, Lindstrom AB, Strynar MJ (2006) Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicol Sci 90(2):510–518
Li K, Gao P, Xiang P, Zhang X, Cui X, Ma LQ (2017a) Molecular mechanisms of PFOA-induced toxicity in animals and humans: implications for health risks. Environ Int 99:43–54
Li K, Sun J, Yang J, Roberts SM, Zhang X, Cui X, Wei S, Ma LQ (2017b) Molecular mechanisms of perfluorooctanoate-induced hepatocyte apoptosis in mice using proteomic techniques. Environ Sci Technol 51(19):11380–11389
Lindstrom AB, Strynar MJ, Libelo EL (2011) Polyfluorinated compounds: past, present, and future. Environ Sci Technol 45(19):7954–7961
Liu H, Wang J, Sheng N, Cui R, Pan Y (2017) Acot1 is a sensitive indicator for PPARα activation after perfluorooctanoic acid exposure in primary hepatocytes of Sprague-Dawley rats. Toxicol in Vitro 42:299–307
Macon MB, Villanueva LR, Tatum-Gibbs K (2011) Prenatal perfluorooctanoic acid exposure in CD-1 mice: low-dose developmental effects and internal dosimetry. Toxicol Sci 122(1):134–145
Mashek DG, Li LO, Coleman RA (2007) Long-chain acyl-CoA synthetases and fatty acid channeling. Futur Lipidol 2(4):465–476
Misra P, Viswakarma N, Reddy JK (2013) Peroxisome proliferator-activated receptor-α signaling in hepatocarcinogenesis. Subcell Biochem 69:77–99
Nakamura MT, Yudell BE, Loor JJ (2014) Regulation of energy metabolism by long-chain fatty acids. Prog Lipid Res 53(1):124–144
Niu Y, Desmarais TL, Tong Z, Yao Y, Costa M (2015) Oxidative stress alters global histone modification and DNA methylation. Free Rad Biol Med 82:22–28
Okamoto M, Reddy JK, Oyasu R (1997) Tumorigenic conversion of a non-tumorigenic rat urothelial cell line by overexpression of H2O2-generating peroxisomal fatty acyl-coa oxidase. Int J Cancer 70(6):716–721
Olson D, Sleiman S, Bourassa M, Wagner FF, Gale JP, Zhang YL, Ratan RR, Holson EB (2015) Hydroxamate-based histone deacetylase inhibitors can protect neurons from oxidative stress via a histone deacetylase-independent catalase-like mechanism. Chem Biol 22(4):439–445
Peng S, Yan L, Zhang J, Wang Z, Tian M, Shen H (2013) An integrated metabonomics and transcriptomics approach to understanding metabolic pathway disturbance induced by perfluorooctanoic acid. J Pharm Biomed Anal 86(4):56–64
Pyper SR, Navin V, Yu S, Reddy JK (2010) PPARα: energy combustion, hypolipidemia, inflammation and cancer. Nucl Recept Signal 8:e002
Quist EM, Filgo AJ, Cummings CA, Kissling GE, Hoenerhoff MJ, Fenton SE (2015) Hepatic mitochondrial alteration in CD-1 mice associated with prenatal exposures to low doses of perfluorooctanoic acid (PFOA). Toxicol Pathol 43(4):546–557
Rakhshandehroo M, Hooiveld G, Muller M, Kersten S (2009) Comparative analysis of gene regulation by the transcription factor PPARalpha between mouse and human. Plos One 4(8):e6796
Rakhshandehroo M, Knoch B, Muller M, Kersten S (2010) Peroxisome proliferator-activated receptor alpha target genes. PPAR Research 2010:1–20. https://doi.org/10.1155/2010/612089
Ricciardi MR, Mirabilii S, Allegretti M, Licchetta R, Calarco A, Torrisi MR, Foà R, Nicolai R, Peluso G, Tafuri A (2015) Targeting the leukemia cell metabolism by the CPT1a inhibition: functional preclinical effects in leukemias. Blood 126(16):1925–1929
Schieber M, Chandel NS (2014) ROS function in redox signaling and oxidative stress. Curr Biol 24(10):R453–R462
Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, Grueter CA, Lim H, Saunders LR, Stevens RD, Newgard CB, Farese RV Jr, de Cabo R, Ulrich S, Akassoglou K, Verdin E (2013) Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 339(6116):211–214
Spiegel S, Milstien S, Grant S (2012) Endogenous modulators and pharmacological inhibitors of histone deacetylases in cancer therapy. Oncogene 31(5):537–551
Tsuda S (2016) Differential toxicity between perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA). J Toxicol Sci 41(Special):SP27–SP36
Wang L, Wang Y, Liang Y et al (2014) PFOS induced lipid metabolism disturbances in balb/c mice through inhibition of low density lipoproteins excretion. Sci Rep 4(1):4582
Wang QW, Yang GP, Zhang ZM, Zhang J (2018) Optimization of sample preparation and chromatography for the determination of perfluoroalkyl acids in sediments from the Yangtze Estuary and East China Sea. Chemosphere 205:524–530
White SS, Calafat AM, Kuklenyik Z, Villanueva L, Zehr RD, Helfant L, Strynar MJ, Lindstrom AB, Thibodeaux JR, Wood C, Fenton SE (2007) Gestational PFOA exposure of mice is associated with altered mammary gland development in dams and female offspring. Toxicol Sci 96(1):133–144
Wolf CJ, Fenton SE, Schmid JE, Calafat AM, Kuklenyik Z, Bryant XA, Thibodeaux J, Das KP, White SS, Lau CS, Abbott BD (2007) Developmental toxicity of perfluorooctanoic acid in the CD-1 mouse after cross-foster and restricted gestational exposures. Toxicol Sci 95(2):462–473
Wu LL, Chiou CC, Chang PY, Wu JT (2004) Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clinica Chimica Acta 339(2):1–9
Wu X, Xie G, Xu X, Wu W, Yang B (2018) Adverse bioeffect of perfluorooctanoic acid on liver metabolic function in mice. Environ Sci Pollut Res 25(5):4787–4793
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (No. 31502111) and the Natural Science Foundation of Hebei (No C2016204097).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, D., Zhang, L., Zhang, Y. et al. Maternal exposure to perfluorooctanoic acid (PFOA) causes liver toxicity through PPAR-α pathway and lowered histone acetylation in female offspring mice. Environ Sci Pollut Res 26, 18866–18875 (2019). https://doi.org/10.1007/s11356-019-05258-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05258-z