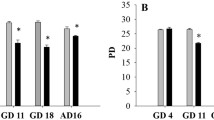
Abstract
Widely used as an antimicrobial in antibacterial bar soaps, triclocarban (3,4,4′-trichlorocarbanilide; TCC) is effective against Gram-positive bacteria but shows little efficacy against Gram-negative strains, potentially altering the composition of indigenous microflora within and on the human body. To date, the consequence of continuous or previous nonprescription antimicrobial exposure from compounds in personal care products on commensal microflora is still elusive. Previous research has shown that TCC exposure during gestation and lactation induced dysbiosis of gut microbial communities among exposed dams and neonates. However, the impact of antimicrobial exposure specifically after discontinuation of the use of TCC on the gut microbiota has not been investigated. In this study, weaned Sprague Dawley rats (postnatal day, PND 22) were provided ad lib access to TCC-supplemented diet (0.2% w/w or 0.5% w/w) for 4 weeks (phase I) followed by a 4-week washout period (phase II) to determine gut microflora changes both during continuous exposure to TCC and to determine the potential rebound following TCC withdrawal. Fecal samples were collected at baseline (PND 22) prior to TCC exposure and throughout phase I and phase II. The V4 region of 16S rDNA was sequenced from extracted total fecal DNA with the MiSeq platform. Exposure to both 0.2% w/w and 0.5% w/w TCC was sufficient to alter diversity of microbiota during phase I of treatment. This effect was further prolonged into phase II, even when TCC exposure was discontinued. Collectively, these data highlight the impact of both continuous and prior TCC exposure on gut microbial ecology and shed light onto the potential long-term health risk of daily nonprescription antimicrobial personal care product use.




Similar content being viewed by others
References
Arrieta MC, Stiemsma LT, Amenyogbe N, Brown EM, Finlay B (2014) The intestinal microbiome in early life: health and disease. Front Immunol 5:427
Aryal N, Reinhold DM (2011) Phytoaccumulation of antimicrobials from biosolids: impacts on environmental fate and relevance to human exposure. Water Res 45:5545–5552
Bokulich NA, Subramanian S, Faith JJ, Gevers D, Gordon JI, Knight R, Mills DA, Caporaso JG (2013) Quality-filtering vastly improves diversity estimates from illumina amplicon sequencing. Nat Methods 10:57–59
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010) Qiime allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336
Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, Gormley N, Gilbert JA, Smith G, Knight R (2012) Ultra-high-throughput microbial community analysis on the illumina hiseq and miseq platforms. ISME J 6:1621–1624
Chen J, Ahn KC, Gee NA, Ahmed MI, Duleba AJ, Zhao L, Gee SJ, Hammock BD, Lasley BL (2008) Triclocarban enhances testosterone action: a new type of endocrine disruptor? Endocrinology 149:1173–1179
Claus SP, Guillou H, Ellero-Simatos S (2016) The gut microbiota: a major player in the toxicity of environmental pollutants? NPJ Biofilms Microbiomes 2:16003
Duleba AJ, Ahmed MI, Sun M, Gao AC, Villanueva J, Conley AJ, Turgeon JL, Benirschke K, Gee NA, Chen J, Green PG, Lasley BL (2011) Effects of triclocarban on intact immature male rat: augmentation of androgen action. Reprod Sci 18:119–127
Duncan SH, Lobley GE, Holtrop G, Ince J, Johnstone AM, Louis P, Flint HJ (2008) Human colonic microbiota associated with diet, obesity and weight loss. Int J Obes 32:1720–1724
Everard A, Lazarevic V, Gaia N, Johansson M, Stahlman M, Backhed F et al (2014) Microbiome of prebiotic-treated mice reveals novel targets involved in host response during obesity. ISME J 8:2116–2130
Food and Drug Administration (2016) Safety and effectiveness of consumer antiseptics; topical antimicrobial drug products for over-the-counter human use. Final rule 0097-6326 (Print)
Frederiksen H, Aksglaede L, Sorensen K, Nielsen O, Main KM, Skakkebaek NE, Juul A, Andersson AM (2013) Bisphenol a and other phenols in urine from danish children and adolescents analyzed by isotope diluted turboflow-lc-ms/ms. Int J Hyg Environ Health 216:710–720
Giuliano CA, Rybak MJ (2015) Efficacy of triclosan as an antimicrobial hand soap and its potential impact on antimicrobial resistance: a focused review. Pharmacotherapy 35:328–336
Hu J, Raikhel V, Gopalakrishnan K, Fernandez-Hernandez H, Lambertini L, Manservisi F, Falcioni L, Bua L, Belpoggi F, L.Teitelbaum S, Chen J (2016) Effect of postnatal low-dose exposure to environmental chemicals on the gut microbiome in a rodent model. Microbiome 4:26
Kennedy RC, Menn FM, Healy L, Fecteau KA, Hu P, Bae J et al (2015) Early life triclocarban exposure during lactation affects neonate rat survival. Reprod Sci 22:75–89
Kennedy RC, Fling RR, Robeson MS, Saxton AM, Donnell RL, Darcy JL, Bemis DA, Liu J, Zhao L, Chen J (2016a) Temporal development of gut microbiota in triclocarban exposed pregnant and neonatal rats. Sci Rep 6:33430
Kennedy RC, Terry PD, Chen J (2016b) Triclocarban and health: the jury is still out. mSphere 1:e00239–e00216
Kindt R, Coe R (2005) Tree diversity analysis: a manual and software for common statistical methods for ecological and biodiversity studies. World Agroforestry Centre, Nairobi PMCid: PMC1156951
Lankelma JM, Belzer C, Hoogendijk AJ, de Vos AF, de Vos WM, van der Poll T, Wiersinga WJ (2016) Antibiotic-induced gut microbiota disruption decreases tnf-alpha release by mononuclear cells in healthy adults. Clin Transl Gastroenterol 7:e186
Lee GC, Reveles KR, Attridge RT, Lawson KA, Mansi IA, Lewis JS 2nd et al (2014) Outpatient antibiotic prescribing in the United States: 2000 to 2010. BMC Med 12:96
Levy SB (2001) Antibacterial household products: cause for concern. Emerg Infect Dis 7:512–515
Lim MY, You HJ, Yoon HS, Kwon B, Lee JY, Lee S, Song YM, Lee K, Sung J, Ko GP (2017) The effect of heritability and host genetics on the gut microbiota and metabolic syndrome. Gut 66:1031–1038
Lindheim L, Bashir M, Munzker J, Trummer C, Zachhuber V, Leber B et al (2017) Alterations in gut microbiome composition and barrier function are associated with reproductive and metabolic defects in women with polycystic ovary syndrome (pcos): a pilot study. PLoS One 12:e0168390
Lippert K, Kedenko L, Antonielli L, Kedenko I, Gemeier C, Leitner M, Kautzky-Willer A, Paulweber B, Hackl E (2017) Gut microbiota dysbiosis associated with glucose metabolism disorders and the metabolic syndrome in older adults. Benef Microbes 8:545–556
McDonnell G, Russell AD (1999) Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev 12:147–179
McMurdie PJ, Holmes S (2013) Phyloseq: an r package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 8:e61217
Mueller NT, Bakacs E, Combellick J, Grigoryan Z, Dominguez-Bello MG (2015) The infant microbiome development: mom matters. Trends Mol Med 21:109–117
Narrowe AB, Albuthi-Lantz M, Smith EP, Bower KJ, Roane TM, Vajda AM, Miller CS (2015) Perturbation and restoration of the fathead minnow gut microbiome after low-level triclosan exposure. Microbiome 3:6
Navas-Molina JA, Peralta-Sanchez JM, Gonzalez A, McMurdie PJ, Vazquez-Baeza Y, Xu Z et al (2013) Advancing our understanding of the human microbiome using qiime. Methods Enzymol 531:371–444
Nolen GA, Dierckman TA (1979) Reproduction and teratogenic studies of a 2:1 mixture of 3,4,4′-trichlorocarbanilide and 3-trifluoromethyl-4,4′-dichlorocarbanilide in rats and rabbits. Toxicol Appl Pharmacol 51:417–425
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D et al (2016) Vegan: Community ecology package. CRAN - Package vegan - CRAN.R-project.org. https://cran.rproject.org/web/packages/vegan/index.html
Pasch E, Voltmer L, Gemmell S, Walter J, Walton KL (2009) Effects of triclosan on the normal intestinal microbiota and on susceptibility to experimental murine colitis. FASEB J 23:977.910–977.910
Perencevich EN, Wong MT, Harris AD (2001) National and regional assessment of the antibacterial soap market: a step toward determining the impact of prevalent antibacterial soaps. Am J Infect Control 29:281–283
Poole AC, Pischel L, Ley C, Suh G, Goodrich JK, Haggerty TD, Ley RE, Parsonnet J (2016) Crossover control study of the effect of personal care products containing triclosan on the microbiome. mSphere 1:e00056–e00015
Prosser RS, Lissemore L, Topp E, Sibley PK (2014) Bioaccumulation of triclosan and triclocarban in plants grown in soils amended with municipal dewatered biosolids. Environ Toxicol Chem 33:975–984
Pycke BF, Geer LA, Dalloul M, Abulafia O, Jenck AM, Halden RU (2014) Human fetal exposure to triclosan and triclocarban in an urban population from Brooklyn, New York. Environ Sci Technol 48:8831–8838
Scharpf LG Jr, Hill ID, Maibach HI (1975) Percutaneous penetration and disposition of triclocarban in man: body showering. Arch Environ Health 30:7–14
Schebb NH, Inceoglu B, Ahn KC, Morisseau C, Gee SJ, Hammock BD (2011) Investigation of human exposure to triclocarban after showering and preliminary evaluation of its biological effects. Environ Sci Technol 45:3109–3115
Sekirov I, Russell SL, Antunes LC, Finlay BB (2010) Gut microbiota in health and disease. Physiol Rev 90:859–904
Sweeney TE, Morton JM (2013) The human gut microbiome: a review of the effect of obesity and surgically induced weight loss. JAMA Surg 148:563–569
Syed AK, Ghosh S, Love NG, Boles BR (2014) Triclosan promotes staphylococcus aureus nasal colonization. MBio 5:e01015
Vangay P, Ward T, Gerber JS, Knights D (2015) Antibiotics, pediatric dysbiosis, and disease. Cell Host Microbe 17:553–564
Velmurugan G, Ramprasath T, Gilles M, Swaminathan K, Ramasamy S (2017) Gut microbiota, endocrine-disrupting chemicals, and the diabetes epidemic. Trends Endocrinol Metab 28:612–625
Wopereis H, Oozeer R, Knipping K, Belzer C, Knol J (2014) The first thousand days - intestinal microbiology of early life: establishing a symbiosis. Pediatr Allergy Immunol 25:428–438
Wu X, Ernst F, Conkle JL, Gan J (2013) Comparative uptake and translocation of pharmaceutical and personal care products (ppcps) by common vegetables. Environ Int 60:15–22
Yassour M, Vatanen T, Siljander H, Hamalainen AM, Harkonen T, Ryhanen SJ et al (2016) Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci Transl Med 8:343ra381
Ye X, Zhou X, Furr J, Ahn KC, Hammock BD, Gray EL, Calafat AM (2011) Biomarkers of exposure to triclocarban in urine and serum. Toxicology 286:69–74
Zaura E, Brandt BW, Teixeira de Mattos MJ, Buijs MJ, Caspers MP, Rashid MU et al (2015) Same exposure but two radically different responses to antibiotics: resilience of the salivary microbiome versus long-term microbial shifts in feces. MBio 6:e01693–e01615
Acknowledgements
The study was conducted at the University of Tennessee Knoxville and supported by the National Institutes of Environmental Health Sciences to Dr. Jiangang Chen (1R21ES017475-01A1). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors and the conduction of experiments adhered to the research ethics guidelines of the journal.
Competing interests
The authors declare that they have no conflicts of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Kennedy, R.C., Fling, R.R., Robeson, M.S. et al. Temporal dynamics of gut microbiota in triclocarban-exposed weaned rats. Environ Sci Pollut Res 25, 14743–14751 (2018). https://doi.org/10.1007/s11356-018-1627-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1627-9