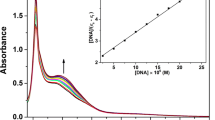
Abstract
Covalent bond formations of free radical metabolites with biomolecules like DNA and proteins are thought to constitute a major mechanism of toxicity and carcinogenesis. Glutathione (GSH) is generally accepted as a radical scavenger protecting the cell. In the present study, we investigated a semiquinone radical (SQ●-) metabolite of the semivolatile 4-chlorobiphenyl, using electron paramagnetic resonance spectroscopy, and oxygen consumption. Proton nuclear magnetic resonance (1H NMR) and liquid chromatography–mass spectrometry (LC-MS) were also employed to elucidate the radical interaction with DNA, amino acids, and GSH. We found that DNA and oligonucleotides stabilized SQ●- by electron delocalization in the π-stacking system, resulting in persistent radical intercalated, rather than forming a covalent bond with SQ●-. This finding was strongly supported by the semiempirical calculation of the semioccupied molecular orbital and the linear combination of the atomic orbitals, indicating 9.8 kcal mol−1 energy gain. The insertion of SQ●- into the DNA strand may result in DNA strand breaks and interruption of DNA replication process or even activate radical mediated secondary reactions. The presence of amino acids resulted in a decrease of the electron paramagnetic resonance (EPR) signal of SQ●- and correlated with their isoelectric points. The pH shifts the equilibrium of the dianions of hydroquinone and influenced indirectly the formation of SQ●-. Similar findings were observed with GSH and Cys. GSH and Cys functioned as indirect radical scavengers; their activities depend on their chemical equilibria with the corresponding quinones, and their further reaction via Michael addition. The generally accepted role of GSH as radical scavenger in biological systems should be reconsidered based upon these findings, questioning the generally accepted view of radical interaction of semiquinones with biologically active compounds, like DNA, amino acids, proteins, and radical scavengers like GSH.







Similar content being viewed by others
Abbreviations
- 4-CB-2’,5’-Q:
-
4-Chlorobiphenyl-2’,5’-benzoquinone
- 4-CB-2’,5’-H2Q:
-
4-Chlorobiphenyl-2’,5’-hydroquinone
- 4-CB-2’,5’-SQ•– :
-
4-Chlorobiphenyl-2’,5’-semiquinone radical
- AA:
-
Arachidonic acid
- DMSO:
-
Dimethyl sulfoxide
- EPR:
-
Electron paramagnetic resonance
- GSH:
-
Glutathione, reduced form
- H2Q:
-
Hydroquinones
- hPGHS-2:
-
Human recombinant prostaglandin H synthase-2
- KAA:
-
Potassium arachidonate
- LCAO:
-
Linear combination of the atomic orbitals
- PCBs:
-
Polychlorinated biphenyls
- Q:
-
Quinones
- ROS:
-
Reactive oxygen species
- SOMO:
-
Semioccupied molecular orbital
- SQ•– :
-
Semiquinone free radicals
References
Amaro AR, Oakley GG, Bauer U, Spielmann HP, Robertson LW (1996) Metabolic activation of PCBs to quinones: reactivity toward nitrogen and sulfur nucleophiles and influence of superoxide dismutase. Chem Res Toxicol 9(3):623–629
Arif JM, Lehmler HJ, Robertson LW, Gupta RC (2003) Interaction of benzoquinone- and hydroquinone-derivatives of lower chlorinated biphenyls with DNA and nucleotides in vitro. Chem Biol Interact 142(3):307–316
Blanksby SJ, Ellison GB (2003) Bond dissociation energies of organic molecules. Acc Chem Res 36(4):255–263
Bolton JL, Thatcher GR (2008) Potential mechanisms of estrogen quinone carcinogenesis. Chem Res Toxicol 21(1):93–101
Bolton JL, Trush MA, Penning TM, Dryhurst G, Monks TJ (2000) Role of quinones in toxicology. Chem Res Toxicol 13(3):135–160
Buettner GR (1993) The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys 300(2):535–543
Davis BK, Beach JK, Wade MJ, Klein AK, Hock K (2002) Risk Assessment of Polychlorinated Biphenyls (PCBs) in Indoor Air
Dwivedy I, Devanesan P, Cremonesi P, Rogan E, Cavalieri E (1992) Synthesis and characterization of estrogen 2,3- and 3,4-quinones. Comparison of DNA adducts formed by the quinones versus horseradish peroxidase-activated catechol estrogens. Chem Res Toxicol 5(6):828–833
Eling TE, Curtis JF, Harman LS, Mason RP (1986) Oxidation of glutathione to its thiyl free radical metabolite by prostaglandin H synthase. A potential endogenous substrate for the hydroperoxidase. J Biol Chem 261(11):5023–5028
Flowers-Geary L, Bleczinki W, Harvey RG, Penning TM (1996) Cytotoxicity and mutagenicity of polycyclic aromatic hydrocarbon ortho-quinones produced by dihydrodiol dehydrogenase. Chem Biol Interact 99(1-3):55–72
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE et al (2004) Gaussian 03. Gaussian, Inc., Wallingford, CT
Harman D (1962) Role of free radicals in mutation, cancer, aging, and the maintenance of life. Radiat Res 16:753–763
Kalyanaraman B, Premovic PI, Sealy RC (1987) Semiquinone anion radicals from addition of amino acids, peptides, and proteins to quinones derived from oxidation of catechols and catecholamines. An ESR spin stabilization study. J Biol Chem 262(23):11080–11087
Kobayashi T, Narumiya S (2002) Function of prostanoid receptors: studies on knockout mice. Prostaglandins Other Lipid Mediat 68-69:557–573
Lehmann L, Esch HL, Kirby PA, Robertson LW, Ludewig G (2007) 4-monochlorobiphenyl (PCB3) induces mutations in the livers of transgenic Fisher 344 rats. Carcinogenesis 28(2):471–478
Li S, Cooper VR, Thonhauser T, Lundqvist BI, Langreth DC (2009) Stacking interactions and DNA intercalation. J Phys Chem B 113(32):11166–11172
Ludewig G, Lehmann L, Esch H, Robertson LW (2008) Metabolic Activation of PCBs to Carcinogens in Vivo - A Review. Environ Toxicol Pharmacol 25(2):241–246
Mill T, Hendry DG, Richardson H (1980) Free-radical oxidants in natural waters. Science 207(4433):886–887
Morrison H, Di Monte D, Nordenskjold M, Jernstrom B (1985) Induction of cell damage by menadione and benzo(a)pyrene-3,6-quinone in cultures of adult rat hepatocytes and human fibroblasts. Toxicol Lett 28(1):37–47
Myers LS (1982) Free radical damages of nucleic acids and their components. In: Pryor WA (ed) Free Radicals in Biology, vol IV. Academic Press, New York, pp 95–115
Nonhebel DC, Walton JC (1974) Free-radical Chemistry. Cambridge University Press, London
Oakley GG, Devanaboyina U, Robertson LW, Gupta RC (1996a) Oxidative DNA damage induced by activation of polychlorinated biphenyls (PCBs): implications for PCB-induced oxidative stress in breast cancer. Chem Res Toxicol 9(8):1285–1292
Oakley GG, Robertson LW, Gupta RC (1996b) Analysis of polychlorinated biphenyl-DNA adducts by 32P-postlabeling. Carcinogenesis 17(1):109–114
O'Brien PJ (1991) Molecular mechanisms of quinone cytotoxicity. Chem Biol Interact 80(1):1–41
Shen L et al (1998) Alkylation of 2'-deoxynucleosides and DNA by the Premarin metabolite 4-hydroxyequilenin semiquinone radical. Chem Res Toxicol 11(2):94–101
Song Y, Buettner GR (2010) Thermodynamic and kinetic considerations for the reaction of semiquinone radicals to form superoxide and hydrogen peroxide. Free Radic Biol Med 49(6):919–962
Song Y, Wagner BA, Lehmler HJ, Buettner GR (2008) Semiquinone radicals from oxygenated polychlorinated biphenyls: electron paramagnetic resonance studies. Chem Res Toxicol 21(7):1359–1367
Srinivasan A, Lehmler HJ, Robertson LW, Ludewig G (2001) Production of DNA strand breaks in vitro and reactive oxygen species in vitro and in HL-60 cells by PCB metabolites. Toxicol Sci 60(1):92–102
Wangpradit O et al (2009) Oxidation of 4-chlorobiphenyl metabolites to electrophilic species by prostaglandin H synthase. Chem Res Toxicol 22(1):64–71
Wangpradit O et al (2010) Observation of an unusual electronically distorted semiquinone radical of PCB metabolites in the active site of prostaglandin H synthase-2. Chemosphere 81(11):1501–1508
Zettner MA et al (2007) Quinoid metabolites of 4-monochlorobiphenyl induce gene mutations in cultured Chinese hamster v79 cells. Toxicol Sci 100(1):88–98
Zhao S, Narang A, Ding X, Eadon G (2004) Characterization and quantitative analysis of DNA adducts formed from lower chlorinated PCB-derived quinones. Chem Res Toxicol 17(4):502–511
Acknowledgments
We would like to thank Brett A. Wagner and Joost van’t Erve, the University of Iowa, for their assistance with EPR and oxygraph measurements. The University of Iowa EPR Facility provided invaluable support. We thank Dr. Lynn M. Teesch, and the high-resolution mass spectrometry facility for HPLC-MS support. This publication was made possible by NIH grant P42 ES 013661 and its training core from the National Institute of Environmental Health Sciences (NIEHS), and by The University of Iowa Environmental Health Sciences Research Center, P30 ES 05605. The project was supported by the Tech For Future fund, an initiative of the Saxion and Windesheim Universities of Applied Sciences and the regional government Overijsel, The Netherlands. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the funding agencies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Wangpradit, O., Rahaman, A., Mariappan, S.V.S. et al. Breaking the dogma: PCB-derived semiquinone free radicals do not form covalent adducts with DNA, GSH, and amino acids. Environ Sci Pollut Res 23, 2138–2147 (2016). https://doi.org/10.1007/s11356-015-5007-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5007-4