Abstract
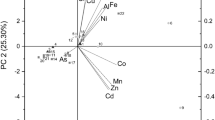
The present study investigates the phenolic profile of exudates and extracts of the green algae Dunaliella tertiolecta, harvested in natural seawater in the absence (control) and in the presence of Cu(II) (315 and 790 nmol L−1) and Fe(III) (900 nmol L−1) in order to identify and quantify the phenolic compounds produced under metallic stress conditions. The presence of metal ions modifies the growth of cells and changes cell metabolism by producing phenolic compounds adapted to the solution. The use of reversed-phase high-performance liquid chromatography (RP-HPLC) permitted the identification of 14 phenolic constituents. The concentration and type of polyphenols detected in cell extracts and in solution are directly related with the metal and its concentration during growth cultures, achieving 1.4 times higher levels of polyphenols under 790 nmol Cu(II) L−1 with respect to the control experiments. Microalga excretes polyphenols to be adapted to the environmental conditions. Gentisic acid, (+) catechin and (−) epicatechin, the most prominent phenolic compounds detected in the algae extracts, showed high antioxidant activity in inhibiting 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals. This potent activity may be related to its presence in cells and exudates in high concentrations.

Similar content being viewed by others
References
Abd El-Baky HH, El Baz FK, El-Baroty GS (2009) Production of phenolic compounds from Spirulina maxima microalgae and its protective effects. Afr J Biotechnol 8(24):7059–7067. doi:10.5897/AJB2009.000-9548
Bondet V, Brand-Williams W, Berset C (1997) Kinetics and mechanisms of antioxidant activity using the DPPH free radical method. Lebensm Wiss Technol 30:609–615. doi:10.1006/fstl.1997.0240
Chacón-Lee TL, González-Mariño GE (2010) Microalgae for “healthy” food- possibilities and challenges. Compr Rev Food Sci Food Saf 9:655–675. doi:10.1111/j.1541-4337.2010.00132.x
Cirulis JT, Scott JA, Ross GM (2013) Management of oxidative stress by microalgae. Can J Physiol Pharmacol 91(1):15–21. doi:10.1139/cjpp-2012-0249
Croot PL, Moffett JW, Brand LE (2000) Production of extracellular Cu complexing ligands by eucaryotic phytoplankton in response to Cu stress. Limnol Oceanogr 45(3):619–627. doi:10.4319/lo.2000.45.3.0619
Dai J, Mumper RJ (2010) Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules 15(10):7313–7352. doi:10.3390/molecules15107313
Dillard CJ, German JB (2000) Phytochemicals: nutraceuticals and human health. J Sci Food Agric 80(12):1744–1756. doi:10.1002/1097-0010(20000915)80:12,1744::AIDJSFA725.3.0.CO;2-W
EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) (2011) Scientific opinion on the reevaluation of butylated hydroxyanisole BHA (E 320) as a food additive. EFSA J 9(10):2392 (49pp)
EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) (2012) Scientific opinion on the reevaluation of butylated hydroxytoluene BHT (E 321) as a food additive. EFSA J 10(3):2588 (43pp)
Gledhill M, van den Berg CMG (1994) Determination of complexation of iron(III) with natural organic complexing ligands in seawater using cathodic stripping voltammetry. Mar Chem 47(1):41–54. doi:10.1016/0304-4203(94)90012-4
González AG, Shirokova LS, Pokrovsky OS, Emnova EE, Martínez RE, Santana-Casiano JM, González-Dávila M, Pokrovski GS (2010) Adsorption of copper on Pseudomonas aureofaciens: protective role of surface expolysaccharides. J Colloid Interface Sci 350(1):305–314
González AG, Santana-Casiano JM, González-Davila M, Pérez N (2012) Effect of organic exudates of Phaeodactylum tricornutum on the Fe(II) oxidation rate constant. Cienc Mar 38:245–261
González-Dávila M, Santana-Casiano JM, Pérez-Peña J, Millero FJ (1995) Binding of Cu(II) to the surface and exudates of the alga Dunaliella tertiolecta in seawater. Environ Sci Technol 29:289–301. doi:10.1021/es00002a004
Guangqiu Q, Chongling Y, Haoliang L (2007) Influence of heavy metals on the carbohydrate and phenolics in mangrove, Aegiceras corniculatum L., seedlings. Bull Environ Contam Toxicol 78(6):440–444. doi:10.1007/s00128-007-9204-9
Guillard MML (1975) Culture of phytoplankton for feeding marine invertebrates. In: Smith WL, Chanley MH (eds) Culture of marine invertebrate animals. Plenum Press, New York, pp 29–60
Imani S, Rezaei-Zarchi S, Hashemi M, Borna H, Javid A, Ali mohamad Zand AM, Abarghouei HB (2011) Hg, Cd and Pb heavy metal bioremediation by Dunaliella alga. J Med Plant Res 5(13):2775–2780
Jones DL (1998) Organic acids in the rhizosphere—a critical review. Plant Soil 205(1):25–44. doi:10.1023/A:1004356007312
Jung C, Maeder V, Funk F, Frey B, Sticher H, Frossard E (2003) Release of phenols from Lupinus albus L. roots exposed to Cu and their possible role in Cu detoxification. Plant Soil 252(1):301–312. doi:10.1023/A:1024775803759
Koukal B, Rosse P, Reinhardt A, Ferraria B, Wilkinson KJ, Loizeau J-L, Dominik J (2007) Effect of Pseudokirchneriella subcapitata (Chlorophyceae) exudates on metal toxicity and colloid aggregation. Water Res 41(1):63–70. doi:10.1016/j.watres.2006.09.014
Lavid N, Schwartz A, Yarden O, Tel-Or E (2001) The involvement of polyphenols and peroxidase activities in heavy-metal accumulation by epidermal glands of the waterlily (Nymphaeaceae). Planta 212(3):323–331. doi:10.1007/s004250000400
Levy JL, Angel BM, Stauber JL, Poon WL, Simpson SL, Cheng SH, Jolley DF (2008) Uptake and internalisation of copper by three marine microalgae: comparison of copper-sensitive and copper-tolerant species. Aquat Toxicol 89(2):82–93. doi:10.1016/j.aquatox.2008.06.003
Michalak A (2006) Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol J Environ Stud 15:523–530
Moreno-Garrido I, Lubián LM, Soares AMVM (2000) Influence of cellular density on determination of EC50 in microalgal growth inhibition tests. Ecotoxicol Environ Saf 47(2):112–116. doi:10.1006/eesa.2000.1953
Munin A, Edwards-Lévy F (2011) Encapsulation of natural polyphenolic compounds; a review. Pharmaceutics 3:793–829. doi:10.3390/pharmaceutics3040793
Nikookar K, Moradshahi A, Hosseini L (2005) Physiological responses of Dunaliella salina and Dunaliella tertiolecta to copper toxicity. Biomol Eng 22:141–146. doi:10.1016/j.bioeng.2005.07.001
Onofrejová L, Vasickova J, Klejdus B, Stratil P, Misurcova L, Kracmar S, Kopecky J, Vacek J (2010) Bioactive phenols in algae: the application of pressurizaed-liquid and solid-phase extraction techniques. J Pharm Biomed Anal 51(2):464–470. doi:10.1016/j.jpba.2009.03.027
Oven M, Grill E, Golan-Goldhirsh A, Kutchan T, Zenk MH (2002) Increase of free cysteine and citric acid in plant cells exposed to cobalt ions. Phytochemistry 60(5):467–474. doi:10.1016/S0031-9422(02)00135-8
Özkoc HB, Taylan ZS (2010) Assessment of various parameters of metal biology in marine microalgae Phaeodactylum tricornutum and Dunaliella tertiolecta. Fresenius Environ Bull 19:29812986
Perales-Vela HV, Peña-Castro JM, Cañizares-Villanueva RO (2006) Heavy metal detoxification in eukaryotic microalgae. Chemosphere 64:1–10. doi:10.1016/j.chemosphere.2005.11.024
Quideau S, Deffieux D, Douat-Casassus C, Pouységu L (2011) Plant polyphenols: chemical properties, biological activities, and synthesis. Angew Chem Int Ed 50:586–621. doi:10.1002/anie.201000044
Reische DW, Lillard DA, Eitenmiller RR (2002) Antioxidants. In: Akoh CC, Min DB (eds) Food lipids: chemistry, nutrition and biotechnology. Marcel Dekker, New York, pp 423–448
Rico M, López A, Santana-Casiano JM, González AG, González-Dávila M (2013) Variability of the phenolic profile in the diatom Phaeodactylum tricornutum growing under copper and iron stress. Limnol Oceanogr 58(1):144–152. doi:10.4319/lo.2013.58.1.0144
Rose AL, Waite TD (2003) Kinetic of iron complexation by dissolved natural organic matter in coastal waters. Mar Chem 84(1–2):85–103. doi:10.1016/S0304-4203(03)00113-0
Santana-Casiano JM, González-Dávila M, Rodríguez MJ, Millero FJ (2000) The effect of organic compounds in the oxidation kinetics of Fe(II). Mar Chem 70:211–222. doi:10.1016/S0304-4203(00)00027-X
Santana-Casiano JM, González-Dávila M, González AG, Rico M, López A, Martel A (2014) Characterization of polyphenol exudates from Phaeodactylum tricornutum and their effects on the chemistry of Fe(II)-Fe(III). Mar Chem 158:10–16. doi:10.1016/j.marchem.2013.11.001
Suresh K, Subramanyam C (1998) Polyphenols are involved in copper binding to cell walls of Neurospora crassa. J Inorg Biochem 69:209–215. doi:10.1016/S0162-0134(97)10001-0
Van den Berg CMG (1995) Evidence for organic complexation of iron in seawater. Mar Chem 50:139–157. doi:10.1016/0304-4203(95)00032-M
Wang C, Lu J, Zhang S, Wang P, Hou J, Qian J (2011) Effects of Pb stress on nutrient uptake and secondary metabolism in submerged macrophyte Vallisneria natans. Ecotoxicol Environ Saf 74(5):1297–1303. doi:10.1016/j.ecoenv.2011.03.005
Wells ML, Trick CG, Cochlan WP, Hughes MP, Trainer VL (2005) Domoic acid: the synergy of iron, copper, and the toxicity of diatoms. Limnol Oceanogr 50(6):1908–1917. doi:10.4319/lo.2005.50.6.1908
Worms I, Simon DF, Hassler CS, Wilkinson KJ (2006) Bioavailability of trace metals to aquatic microorganisms: importance of chemical, biological and physical processes on biouptake. Biochimie 88(11):1721–1731. doi:10.1016/j.biochi.2006.09.008
Wu J, Luther GW (1995) Complexation of Fe(III) by natural organic ligands in the Northwest Atlantic Ocean by a competitive ligand equilibration method and a kinetic approach. Mar Chem 50:159–177. doi:10.1016/j.biochi.2006.09.008
Acknowledgments
This study received financial support from the Project CTM2010-19517-MAR financed by the Ministerio de Economía y Competitividad in Spain. Our thanks to the Spanish Bank of Algae (BEA) in Gran Canaria for providing algal strains.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
López, A., Rico, M., Santana-Casiano, J.M. et al. Phenolic profile of Dunaliella tertiolecta growing under high levels of copper and iron. Environ Sci Pollut Res 22, 14820–14828 (2015). https://doi.org/10.1007/s11356-015-4717-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4717-y