Abstract
Purpose
The orexigenic peptides, ghrelin, galanin, and orexin-A, have an important role in food intake and energy homeostasis and regulate the higher brain functions including the sleep–wake state. Although the interactions of these neuropeptides affect neuroendocrine systems resulting in obesity, a major risk factor for obstructive sleep apnea syndrome (OSAS), the mechanism has not been fully elucidated. The objective of this study was to evaluate the association of serum ghrelin, galanin, and orexin-A levels with OSAS.
Methods
In this cross-sectional study, patients who underwent one-night polysomnography and conformed to the inclusion criteria were asked to participate. A blood sample was obtained from all participants on the morning of the sleep test to evaluate the serum levels of ghrelin, galanin, and orexin-A using the enzyme-linked immunosorbent assay (ELISA) method. Demographic characteristics, polysomnography data, and serum levels of the participants were recorded and analyzed. Comparison between the OSAS groups was performed by independent sample t-test, Mann–Whitney U test, and Kruskal–Wallis test with post hoc K-W test using SPSS 20.0.
Results
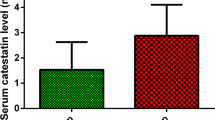
Of 272 patients, those in the OSAS group (n=210) were older than patients in the non-OSAS group (n=62), p < 0.003, and had increased BMI, p < 0.006. Patients with, serum ghrelin, galanin, and orexin-A levels were significantly elevated in patients with OSAS (635.9 pg/mL vs. 420.7 pg/mL, 91.0 pg/mL vs. 60.0 pg/mL, 600.3 pg/mL vs. 485.6 pg/mL, respectively) and found to be higher in patients with severe OSAS than mild and moderate cases (p < 0.01). In multinomial logistic regression to predict the OSAS severity, levels of serum ghrelin (OR = 1.016 [1.010–1.021]; p < 0.001), galanin (OR = 1.050 [1.020–1.081]; p < 0.001), and orexin-A (OR = 1.021 [1.012–1.030]; p < 0.001) were significantly associated only with a moderate level of OSAS.
Conclusion
The orexigenic neuropeptides were found to be an independent determinant of the presence of OSAS and correlate with the severity of OSAS. Increased levels of ghrelin, galanin, and orexin-A were associated with the presence of moderate OSAS.


Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Strollo PJ, Rogers RM (1996) Obstructive sleep apnea. NEJM 334(2):99–104
Kapur V, Blough DK, Sandblom RE, Hert R, de Maine JB, Sullivan SD, Psaty BM (1999) The medical cost of undiagnosed sleep apnea. Sleep 22(6):749–755
Punjabi NM (2008) The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc 5(2):136–143
Sun ML, Niu X, Xiao XY, Chen X. The differences in plasma/serum ghrelin levels between obstructive sleep apnea-hypopnea patients and controls: a protocol for systematic review and meta-analysis. Medicine 2021;100:8(e24368).
Jehan S, Zizi F, Pandi-Perumal SR, Wall S, Auguste E, Myers AK, Jean-Louis G, McFarlane SI (2017) Obstructive sleep apnea and obesity: implications for public health. Sleep Med Disord 1(4):00019
Hamilton GS, Joosten SA (2017) Obstructive sleep apnoea and obesity. Aust Fam Physician 46(7):460–463
Akalu Y, Molla MD, Dessie G, Ayelign B. Physiological effect of ghrelin on body systems. Int J Endocrinol.2020:1385138.
Stoyanova II. Ghrelin: a link between aging, metabolism and neurodegenerative disorders. Neurobiol Dis. 2014;72 Pt A:72–83.
Mechenthaler I (2008) Galanin and the neuroendocrine axes. Cell Mol Life Sci 65(12):1826–1835
Marcos P, Coveñas R (2021) Neuropeptidergic control of feeding: focus on the galanin family of peptides. Int J Mol Sci 22(5):2544
Ma Y, Miracca G, Yu X, Harding EC, Miao A, Yustos R, Vyssotski AL, Franks NP, Wisden W (2019) Galanin neurons unite sleep homeostasis and α2-adrenergic sedation. Curr Biol 29(19):3315-3322.e3
Kroeger D, Absi G, Gagliardi C, Bandaru SS, Madara JC, Ferrari LL, Arrigoni E, Münzberg H, Scammell TE, Saper CB, Vetrivelan R (2018) Galanin neurons in the ventrolateral preoptic area promote sleep and heat loss in mice. Nat Commun 9(1):4129
Mieda M (2017) The roles of orexins in sleep/wake regulation. Neurosci Res 118:56–65
Messina G, Monda V, Moscatelli F, Valenzano AA, Monda G, Esposito T et al (2015) Role of orexin system in obesity. Biol Med (Aligarh) 7:248
Ohno K, Sakurai T (2008) Orexin neuronal circuitry: role in the regulation of sleep and wakefulness. Front Neuroendocrinol 29(1):70–87
World Health Organization: obesity and overweight. WHO Fact Sheet No. 311, Geneva:WHO; 2015.
Berry RB, Gamaldo CE, Harding SM, Brooks R, Lloyd RM, Vaughn BV, Marcus CL. AASM scoring manual version 2.2 updates: new chapters for scoring infant sleep staging and home sleep apnea testing. J Clin Sleep Med. 2015.
Allan HJ (1969) A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Electroenceph clin Neurophysiol 20:644
Thorpy MJ (2012) Classification of sleep disorders. Neurotherapeutics 9:687–701
West SD, Turnbull C (2018) Obstructive sleep apnoea. Eye (Lond) 32(5):889–903
Mano M, Hoshino T, Sasanabe R, Murotani K, Nomura A, Hori R, Konishi N, Baku M, Shiomi T (2019) Impact of gender and age on rapid eye movement-related obstructive sleep apnea: a clinical study of 3234 Japanese OSA patients. Int J Environ Res Public Health 16(6):1068
Kuvat N, Tanriverdi H, Armutcu F (2020) The relationship between obstructive sleep apnea syndrome and obesity: a new perspective on the pathogenesis in terms of organ crosstalk. Clin Respir J 14(7):595–604
Ciavarella D, Tepedino M, Chimenti C, Troiano G, Mazzotta M, Foschino Barbaro MP, Lo Muzio L, Cassano M (2018) Correlation between body mass index and obstructive sleep apnea severity indexes - a retrospective study. Am J Otolaryngol 39(4):388–391
Bikov A, Lazar Z, Horvath P, Tarnoki DL, Tarnoki AD, Fesus L, Horvath M, Meszaros M, Losonczy G, Kunos L (2019) Association between serum lipid profile and obstructive respiratory events during REM and non-REM sleep. Lung 197(4):443–450
Feingold KR. Obesity and dyslipidemia. 2020 Nov 2. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, Dungan K, Grossman A, Hershman JM, Hofland J, Kalra S, Kaltsas G, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, McGee EA, McLachlan R, Morley JE, New M, Purnell J, Sahay R, Singer F, Stratakis CA, Trence DL, Wilson DP, editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000–.
Martínez-Cerón E, Casitas R, Galera R, Sánchez-Sánchez B, Zamarrón E, Garcia-Sanchez A, Jaureguizar A, Cubillos-Zapata C, Garcia-Rio F (2021) Contribution of sleep characteristics to the association between obstructive sleep apnea and dyslipidemia. Sleep Med 84:63–72
Ryan S, Crinion SJ, McNicholas WT (2014) Obesity and sleep-disordered breathing–when two “bad guys” meet. QJM 107(12):949–954
Myers MG Jr, Leibel RL, Seeley RJ, Schwartz MW (2010) Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab 21(11):643–651
De Vriese C, Delporte C (2008) Ghrelin: A new peptide regulating growth hormone release and food intake. Int J Biochem Cell Biol 40(8):1420–1424
Fagerberg B, Hultén LM, Hulthe J (2003) Plasma ghrelin, body fat, insulin resistance, and smoking in clinically healthy men: the atherosclerosis and insulin resistance study. Metabolism 52(11):1460–1463
Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura SA (2001) Role for ghrelin in the central regulation of feeding. Nature 409(6817):194–198
Nieminen P, Mustonen A-M (2004) Effects of peripheral ghrelin on the carbohydrate and lipid metabolism of the tundra vole (Microtus oeconomus). Gen Comp Endocrinol 138:182–187
De Santis S, Cambi J, Tatti P, Bellussi L, Passali D (2015) Changes in ghrelin, leptin and pro-inflammatory cytokines after therapy in Obstructive Sleep Apnea Syndrome (OSAS) patients. Otolaryngol Pol 69(2):1–8
Ulukavak Ciftci T, Kokturk O, Bukan N, Bilgihan A (2005) Leptin and ghrelin levels in patients with obstructive sleep apnea syndrome. Respiration 72(4):395–401
Harsch IA, Konturek PC, Koebnick C, Kuehnlein PP, Fuchs FS, Pour Schahin S, Wiest GH, Hahn EG, Lohmann T, Ficker JH (2003) Leptin and ghrelin levels in patients with obstructive sleep apnoea: effect of CPAP treatment. Eur Respir J 22:251–257
Ursavas A, Ilcol YO, Nalci N, Karadag M, Ege E (2010) Ghrelin, leptin, adiponectin, and resistin levels in sleep apnea syndrome: role of obesity. Ann Thorac Med 5(3):161–165
Sartin JL, Dyer C, Matteri R, Buxton D, Buonomo F, Shores M, Baker J, Osborne JA, Braden T, Steele B (2001) Effect of intracerebroventricular orexin-B on food intake in sheep. J Anim Sci 79(6):1573–1577
Nixon JP, Mavanji V, Butterick TA, Billington CJ, Kotz CM, Teske JA (2015) Sleep disorders, obesity, and aging: the role of orexin. Ageing Res Rev 20:63–73
Michael NJ, Elmquist JK (2020) Coordination of metabolism, arousal, and reward by orexin/hypocretin neurons. J Clin Invest 130(9):4540–4542
Acknowledgements
We would like to thank Assoc. Prof. Dr. Adnan Karaibraihimoğlu (faculty member of the Department of Biostatistics and Medical Informatics) for contributing to the correction of the statistical analyses in our study.
Funding
This study was funded by Süleyman Demirel University Fund (Project No: 4422-YL2-15).
Author information
Authors and Affiliations
Contributions
Önder Öztürk conceived and designed the study. Defne Cebeci, Eda Evgen Tülüceoğlu, and Taner Gonca participated in the acquisition of data. Önder Öztürk and Taner Gonca conducted the scoring of polysomnography. Uğur Şahin and Defne Cebeci analyzed the data. Önder Öztürk drafted the article. Nilüfer Şahin Calapoğlu and Mustafa Calapağlu revised the article critically for important intellectual content. All authors read and approved the version to be submitted.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the Ethics Committee of Süleyman Demirel University Faculty of Medicine (meeting date, July 09, 2015; the number of decisions, 139). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Conflicting interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Öztürk, Ö., Cebeci, D., Şahin, U. et al. Circulating levels of ghrelin, galanin, and orexin-A orexigenic neuropeptides in obstructive sleep apnea syndrome. Sleep Breath 26, 1209–1218 (2022). https://doi.org/10.1007/s11325-021-02514-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-021-02514-w