Abstract
Purpose
To investigate the effect of 4-phenylbutyric acid (4-PBA) on intermittent hypoxia (IH)-induced liver cell injury and to clarify the underlying mechanisms.
Methods
L02 cells (normal human liver cells) were cultured in normoxic condition or subjected to intermittent hypoxia for 4, 8, and 12 h. A part of hypoxia-treated L02 cells was applied with 4-PBA 1 h before exposure to hypoxia. The effect of 4-PBA on liver injury, hepatocyte apoptosis, endoplasmic reticulum stress (ERS), and PERK-eIFa2-ATF4-CHOP apoptotic pathway was investigated.
Results
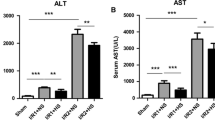
(1) IH caused apoptosis in hepatocyte; (2) IH caused ERS in hepatocyte; (3) IH caused hepatic injury; (4) 4-PBA attenuated IH-induced liver cell injury; (5) 4-PBA protected liver cell from IH-induced apoptosis; (6) 4-PBA suppressed ERS-related apoptotic pathway (PERK-eIFa2-ATF4-CHOP), but did not suppress IH-induced unfold protein reaction (UPR).
Conclusions
4-PBA could protect liver cells by suppressing IH-induced apoptosis mediated by ERS, but not by reducing the UPR.




Similar content being viewed by others
Abbreviations
- 4-PBA:
-
4-phenylbutyric acid
- ALT:
-
alanine aminotransferase
- AST:
-
aspartate aminotransferase
- ATF4:
-
activating transcription Factor 4
- CHOP:
-
C/EBP homologous protein
- ERS:
-
endoplasmic reticulum stress
- FBS:
-
fetal bovine serum
- GRP78:
-
glucose-regulated protein 78
- IH:
-
intermittent hypoxia
- NC:
-
negative control
- OSA:
-
obstructive sleep apnea
- PBS:
-
phosphate-buffered saline
- p-eIF2α:
-
phosphorylated-eukaryotic translation initiation factor 2 subunit α
- PMSF:
-
phenylmethanesulphonylfluoride
- p-PERK:
-
phosphorylated-PKR-like ER protein kinase
- UPR:
-
unfold protein reaction
References
Punjabi NM, Caffo BS, Goodwin JL et al (2009) Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med 6:e1000132
Lévy P, Tamisier R, Minville C, Launois S, Pépin J-L (2011) Sleep apnoea syndrome in 2011: current concepts and future directions. Eur Respir Rev 20:134–146
Tatsumi K, Saibara T (2005) Effects of obstructive sleep apnea syndrome on hepatic steatosis and nonalcoholic steatohepatitis. Hepatol Res 33:100–104
Chou TC, Liang WM, Wang CB, Wu TN, Huang LW (2015) Obstructive sleep apnea is associated with liver disease: a population-based cohort study. Sleep Med 16:955–960
Lin QC, Chen LD, Chen GP, Zhao JM, Chen X, Huang JF, Wu LH (2015) Association between nocturnal hypoxia and liver injury in the setting of nonalcoholic fatty liver disease. Sleep Breath 19:273–280
Aron-Wisnewsky J, Minville C, Tordjman J, Lévy P, Bouillot JL, Basdevant A, Bedossa P, Clément K, Pépin JL (2012) Chronic intermittent hypoxia is a major trigger for non-alcoholic fatty liver disease in morbid obese. J Hepatol 56:225–233
Lemoine M, Serfaty L (2012) Chronic intermittent hypoxia: a breath of fresh air in the understanding of NAFLD pathogenesis. J Hepatol 56:20–22
Ding W, Zhang X, Huang H, Ding N, Zhang S, Hutchinson SZ, Zhang X (2014) Adiponectin protects rat myocardium against chronic intermittent hypoxia-induced injury via inhibition of endoplasmic reticulum stress. PLoS One 9:e94545
Cai XH, Li XC, Jin SW, Liang DS, Wen ZW, Cao HC, Mei HF, Wu Y, Lin ZD, Wang LX (2014) Endoplasmic reticulum stress plays critical role in brain damage after chronic intermittent hypoxia in growing rats. Exp Neurol 257:148–156
Zhou S, Yin X, Zheng Y, Miao X, Feng W, Cai J, Cai L (2014) Metallothionein prevents intermittent hypoxia-induced cardiac endoplasmic reticulum stress and cell death likely via activation of Akt signaling pathway in mice. Toxicol Lett 227:113–123
Kapoor A, Sanyal AJ (2009) Endoplasmic reticulum stress and the unfolded protein response. Clin Liver Dis 4:581–590
Walter P, Ron D (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 6059:1081–1086
Sun L, Liu N, Liu SS et al (2015) Beclin-1 independent autophagy mediates programmed cancer cell death through interplays with endoplasmic reticulum and/or mitochondria in colbat chloride-induced hypoxia. Am J Cancer Res 9:2626–2642
Blais JD, Addison CL, Edge R et al (2006) Perk-dependent translational regulation promotes tumor cell adaptation and angiogenesis in response to hypoxic stress. Mol Cell Biol 24:9517–9532
van den Beucken T, Magagnin MG, Jutten B et al (2011) Translational control is a major contributor to hypoxia induced gene expression. Radiother Oncol 3:379–384
Malhi H, Kaufman RJ (2011) Endoplasmic reticulum stress in liver disease. J Hepatol 54:795–809
Kaplowitz N, Than TA, Shinohara M, Ji C (2007) Endoplasmic reticulum stress and liver injury. Semin Liver Dis 27:367–377
Kaplowitz N, Ji C (2006) Unfolding new mechanisms of alcoholic liver disease in the endoplasmic reticulum. J Gastroenterol Hepatol 21(Suppl 3):S7–S9
Ji C (2006) Dissection of endoplasmic reticulum stress signaling in alcoholic and non-alcoholic liver injury. J Gastroenterol Hepatol 23(Suppl 1):S16–S24
Zhang XQ, Xu CF, Yu CH, Li YM (2014) Role of endoplasmic reticulum stress in the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol 7:1768–1776
Chang C, Tsai H, Chiang M, Huang PL, Shyue SK, Chau LY (2015) TRC8 downregulation contributes to the development of non-alcoholic steatohepatitis by exacerbating hepatic endoplasmic reticulum stress. Biochim Biophys Acta 11:2339–2351
Hetz C (2012) The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 13:89–102
Grimm S (2012) The ER-mitochondria interface: the social network of cell death. Biochim Biophys Acta 1823:327–334
Shore GC, Papa FR, Oakes SA (2011) Signaling cell death from the endoplasmic reticulum stress response. Curr Opin Cell Biol 23:143–149
Ayala P, Montenegro J, Vivar R, Letelier A, Urroz PA, Copaja M, Pivet D, Humeres C, Troncoso R, Vicencio JM, Lavandero S, Díaz-Araya G (2012) Attenuation of endoplasmic reticulum stress using the chemical chaperone 4-phenylbutyric acid prevents cardiac fibrosis induced by isoproterenol. Exp Mol Pathol 92:97–104
Park CS, Cha H, Kwon EJ, Sreenivasaiah PK, Kim DH (2012) The chemical chaperone 4-phenylbutyric acid attenuates pressure-overload cardiac hypertrophy by alleviating endoplasmic reticulum stress. Biochem Biophys Res Commun 421:578–584
He Y, Long J, Zhong W, Fu Y, Li Y, Lin S (2014) Sustained endoplasmic reticulum stress inhibits hepatocyte proliferation via downregulation of c-Met expression. Mol Cell Biochem 389:151–158
Benotti P, Wood GC, Argyropoulos G, Pack A, Keenan BT, Gao X, Gerhard G, Still C (2016) The impact of obstructive sleep apnea on nonalcoholic fatty liver disease in patients with severe obesity. Obesity (Silver Spring) 24:871–877
Chen X, Lin X, Chen LD, Lin QC, Chen GP, Yu YH, Huang JC, Zhao JM (2016) Obstructive sleep apnea is associated with fatty liver index, the index of nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol 28:650–655
Cakmak E, Duksal F, Altinkaya E et al (2015) Association between the severity of nocturnal hypoxia in obstructive sleep apnea and non-alcoholic fatty liver damage. Hepat Mon 15:e32655
Savransky V, Nanayakkara A, Vivero A et al (2007) Chronic intermittent hypoxia predisposes to liver injury. Hepatology 4:1007–1013
Dogan A, Sabri K, Kursat MO (2015) Evaluation of liver functions based on serum aminotransferase enzyme levels in patients with obstructive sleep apnea syndrome. Indian J Otolaryngol Head Neck Surg 12:1–4
Daltro C, Cotrim HP, Alves E, de Freitas LA, Araújo L, Boente L, Leal R, Portugal T (2010) Nonalcoholic fatty liver disease associated with obstructive sleep apnea: just a coincidence? Obes Surg 20:1536–1543
Byrne TJ, Parish JM, Somers V, Aqel BA, Rakela J (2010) Evidence for liver injury in the setting of obstructive sleep apnea. Ann Hepatol 11:228–231
Tanne F, Gagnadoux F, Chazouilleres O, et a1 (2015) Chronic liver injury during obstructive sleep apnea. Hepatology 41:1290–1296
Norman D, Bardwell WA, Arosemena F et al (2008) Serum aminotransferase levels are associated with markers of hypoxia in patients with obstructive sleep apnea. Sleep 31:121–126
Sookoian S, Pirola CJ (2013) Obstructive sleep apnea is associated with fatty liver and abnormal liver enzymes: a meta-analysis. Obes Surg 23:1815–1825
Lo Monaco A, Tobaldini E, Bulgheroni M, Pecis M, Stracuzzi M, Pulixi E, Pelusi S, Porzio M, Fracanzani L, Fargion S, Valenti L, Montano N (2015) Obstructive sleep apnea syndrome in non severely obese patients with nonalcoholic fatty liver disease. Auton Neurosci 192:107–108
Li J, Zhang YL, Chen R et al (2015) Elevated serum liver enzymes in patients with obstructive sleep apnea-hypopnea syndrome. Chin Med J 22:2983–2987
Zhang W, Xu J (2018) Adaptive unfolded protein response promotes cell survival in rifampicin-treated L02 cells. Int J Mol Med 4:2233–2242
Nissar AU, Sharma L, Mudasir MA et al (2017) Chemical chaperone 4-phenyl butyric acid (4-PBA) reduces hepatocellular lipid accumulation and lipotoxicity through induction of autophagy. J Lipid Res 9:1855–1868
Guo HL, Hassan HM, Ding PP, Wang SJ, Chen X, Wang T, Sun LX, Zhang LY, Jiang ZZ (2017) Pyrazinamide-induced hepatotoxicity is alleviated by 4-PBA via inhibition of the PERK-eIF2α-ATF4-CHOP pathway. Toxicology 378:65–75
Ding W, Cai Y, Wang W, Ji L, Dong Y, Zhang X, Su M, Liu J, Lu G, Zhang X (2016) Adiponectin protects the kidney against chronic intermittent hypoxia-induced injury through inhibiting endoplasmic reticulum stress. Sleep Breath 20:1069–1074
Pai P, Lai CJ, Lin CY, Liou YF, Huang CY, Lee SD (2016) Effect of superoxide anion scavenger on rat hearts with chronic intermittent hypoxia. J Appl Physiol (1985) 120:982–990
Chen HL, Lu CH, Lin HC et al (2015) White matter damage and systemic inflammation in obstructive sleep apnea. Sleep 38:361–370
Luedde T, Kaplowitz N, Schwabe RF (2014) Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology 147:765–783
Zhen YQ, Wu YM, Sang YH, Wang Y, Song QY, Yu L, Rao XJ, Dong RH (2015) 2,3-Oxidosqualene cyclase protects liver cells from the injury of intermittent hypoxia by regulating lipid metabolism. Sleep Breath 19:1475–1481
Gorman AM, Healy SJ, Jager R, Samali A (2012) Stress management at the ER: regulators of ER stress-induced apoptosis. Pharmacol Ther 134:306–316
Kim R, Emi M, Tanabe K, Urakami S (2006) Role of the unfolded protein response in cell death. Apoptosis 11:5–13
Xie Q, Khaoustov VI, Chung CC et al (2002) Effect of tauroursodeoxycholic acid on endoplasmic reticulum stress-induced caspase-12 activation. Hepatology 3:592–601
Gu LL, Shen ZL, Li YL, Bao YQ, Lu H (2018) Oxymatrine causes hepatotoxicity by promoting the phosphorylation of JNK and induction of endoplasmic reticulum stress mediated by ROS in L02 cells. Mol Cell 5:401–412
Xu LH, Xie H, Shi ZH, du LD, Wing YK, Li AM, Ke Y, Yung WH (2015) Critical role of endoplasmic reticulum stress in chronic intermittent hypoxia-induced deficits in synaptic plasticity and long-term memory. Antioxid Redox Signal 23:695–710
Lee AS (2005) The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic stress. Methods 4:373–381
Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8:519–529
Tabas I, Ron D (2011) Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol 13:184–190
Rao J, Zhang C, Wang P et al (2015) C/EBP homologous protein (CHOP) contributes to hepatocyte death via the promotion of ERO1alpha signalling in acute liver failure. Biochem J 2:369–378
Li X, Wang Y, Wang H, Huang C, Huang Y, Li J (2015) Endoplasmic reticulum stress is the crossroads of autophagy, inflammation, and apoptosis signaling pathways and participates in liver fibrosis. Inflamm Res 1:1–7
Lee WS, Yi SM, Yun JW, Jung JH, Kim DH, Kim HJ, Chang SH, Kim GS, Ryu CH, Shin SC, Hong SC, Choi YH, Jung JM (2014) Polyphenols isolated from Allium cepa L. induces apoptosis by induction of P53 and suppression of Bcl-2 through inhibiting PI3K/Akt signaling pathway in AGS human cancer cells. J Cancer Prev 19:14–22
Gaudette BT, Iwakoshi NN, Boise LH (2014) Bcl-xL protein protects from C/EBP homologous protein (CHOP)-dependent apoptosis during plasma cell differentiation. J Biol Chem 289:23629–23640
Yang YL, Xiang RL, Sun Q, Feng K, Zhang WL (2016) 4-Phenylbutyric acid attenuates the endoplasmic reticulum stress in type 2 diabetes rats. Basic Clin Med 6:728–733
Funding
National Natural Science Foundation Committee of China provided financial support in the form of National Natural Science Foundation of China funding (No. 81670086, No. 81370183). Natural Science Foundation Committee of Tianjin City provided financial support in the form of Natural Science Foundation of Tianjin City funding (No. 14JCYBJC27800) and major special project for the prevention and control of chronic diseases in Tianjin (Grant No. 17ZXMFSY00080). The sponsor had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
For this type of study formal consent is not required.
Additional information
Comment
This study shows that increasing exposure of hepatocytes to hypoxia demonstrates hypoxia causes a dose-dependent injury which is partly attenuated by 4-phenylbutyric acid. The role of intermittent hypoxia in the pathophysiology of OSA-related liver dysfunction is clearly of interest to scientists and clinicians alike; this study helps advance our understanding of this area and potential therapeutic pathways.
While this is an in vitro study, it does provide support for the notion that currently accepted adequate notions of treatment efficacy may need to be reconsidered. Perhaps with CPAP, some is helpful, more is better, and most of the night is better still.
Ian Wilcox
NSW, Australia
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xin, L., Fan, W., Tingting, D. et al. 4-phenylbutyric acid attenuates endoplasmic reticulum stress-mediated apoptosis and protects the hepatocytes from intermittent hypoxia-induced injury. Sleep Breath 23, 711–717 (2019). https://doi.org/10.1007/s11325-018-1739-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-018-1739-y