Abstract
Purpose
Scintigraphic imaging of malignant glioblastoma (MG) continues to be challenging. We hypothesized that VPAC1 cell surface receptors can be targeted for positron emission tomography (PET) imaging of orthotopically implanted MG in a mouse model, using a VPAC1-specific peptide [64Cu]TP3805.
Procedures
The expression of VPAC1 in mouse GL261 and human U87 glioma cell lines was determined by western blot. The ability of [64Cu]TP3805 to bind to GL261 and U87 cells was studied by cell-binding. Receptor-blocking studies were performed to validate receptor specificity. GL261 tumors were implanted orthotopically in syngeneic T-bet knockout C57BL/6 mouse brain (N = 15) and allowed to grow for 2–3 weeks. Mice were injected i.v., first with ~ 150 μCi of 2-deoxy-2-[18F]fluoro-D-glucose ([18F]FDG) then 24 h later with ~ 200 μCi of [64Cu]TP3805. In another set of tumor-bearing mice, (N = 5), ionic [64Cu]Cl2 was injected as a control. Mice were imaged at a 2-h post-injection using an Inveon micro-PET/CT, sacrificed and % ID/g of [64Cu]TP3805 and [64Cu]Cl2 were calculated in a tumor, normal brain, and other tissues. For histologic tissue examination, 3-μm thick sections of the tumors and normal brain were prepared, digital autoradiography (DAR) was performed, and then the sections were H&E stained for histologic examination.
Results
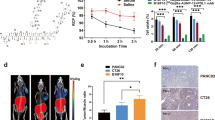
Western blots showed a strong signal for VPAC1 on both cell lines. [64Cu]TP3805 cell-binding was 87 ± 1.5 %. Receptor-blocking reduced cell-binding to 24.3 ± 1.5 % (P < 0.01). PET imaging revealed remarkable accumulation of [64Cu]TP3805 in GL261 MG with a negligible background in the normal brain, as compared to [18F]FDG. Micro-PET/CT image analyses and tissue distribution showed that the brain tumor uptake for [64Cu]TP3805 was 8.2 ± 1.7 % ID/g and for [64Cu]Cl2 2.1 ± 0.5 % ID/g as compared to 1.0 ± 0.3 % ID/g and 1.4 ± 0.3 % ID/g for normal mouse brains, respectively. The high tumor/normal brain ratio for [64Cu]TP3805 (8.1 ± 1.1) allowed tumors to be visualized unequivocally. Histology and [64Cu]TP3805 DAR differentiated malignant tumors from healthy brain and confirmed PET findings.
Conclusion
Targeting VPAC1 receptors using [64Cu]TP3805 for PET imaging of MG is a promising novel approach and calls for further investigation.





Similar content being viewed by others
References
Huse JT, Holland EC (2010) Targeting brain cancer: advances in the molecular pathology of malignant glioma and medulloblastoma. Nat Rev Cancer 10:319–331
Wen PY, Kesari S (2008) Malignant gliomas in adults. N Engl J Med 359:492–507
Krex D, Klink B, Hartmann C, von Deimling A, Pietsch T, Simon M, Sabel M, Steinbach JP, Heese O, Reifenberger G, Weller M, Schackert G, for the German Glioma Network (2007) Long-term survival with glioblastoma multiforme. Brain 130:2596–2606
Scott JN, Brasher PM, Sevick RJ, Rewcastle NB, Forsyth PA (2002) How often are nonenhancing supratentorial gliomas malignant? A population study. Neurology 59:947–949
Yang I, Aghi MK (2009) New advances that enable identification of glioblastoma recurrence. Nat Rev Clin Oncol 6:648–657
Zhou J, Tryggestad E, Wen Z, Lal B, Zhou T, Grossman R, Wang S, Yan K, Fu DX, Ford E, Tyler B, Blakeley J, Laterra J, van Zijl PCM (2011) Differentiation between glioma and radiation necrosis using molecular magnetic resonance imaging of endogenous proteins and peptides. Nat Med 17:130–134
Nikaki A, Angelidis G, Efthimiadou R, Tsougos I, Valotassiou V, Fountas K, Prasopoulos V, Georgoulias P (2017) 18F-fluorothymidine PET imaging in gliomas: an update. Ann Nucl Med 31:495–505
la Fougere C, Suchorska B, Bartenstein P, Kreth FW, Tonn JC (2011) Molecular imaging of gliomas with PET: opportunities and limitations. Neuro-Oncology 13:806–819
Fu Y, Ong LC, Ranganath SH, Zheng L, Kee I, Zhan W, Yu S, Chow PKH, Wang CH (2016) A dual tracer 18F-FCH/18F-FDG PET imaging of an orthotopic brain tumor xenograft model. PLoS One 11:e0148123
Wong TZ, van der Westhuizen GJ, Coleman RE (2002) Positron emission tomography imaging of brain tumors. Neuroimaging Clin N Am 12:615–626
Strauss LG (1996) Fluorine-18 deoxyglucose and false-positive results: a major problem in the diagnostics of oncological patients. Eur J Nucl Med 23:1409–1415
Gallagher BM, Fowler JS, Gutterson NI, MacGregor R, Wan CN, Wolf AP (1978) Metabolic trapping as a principle of oradiopharmaceutical design: some factors resposible for the biodistribution of [18F]2-deoxy-2-fluoro-D-glucose. J Nucl Med 19:1154–1161
Juhasz C, Dwivedi S, Kamson DO et al (2014) Comparison of amino acid positron emission tomographic radiotracers for molecular imaging of primary and metastatic brain tumors. Mol Imaging 13:7290.2014.00015
Szopa W, Burley TA, Kramer-Marek G, Kaspera W (2017) Diagnostic and therapeutic biomarkers in glioblastoma: current status and future perspectives. Biomed Res Int 2017:8013575
Zia H, Hida T, Jakowlew S, Birrer M, Gozes Y, Reubi JC, Fridkin M, Gozes I, Moody TW (1996) Breast cancer growth is inhibited by vasoactive intestinal peptide (VIP) hybrid, a synthetic VIP receptor antagonist. Cancer Res 56:3486–3489
Leyton J, Gozes Y, Pisegna J, Coy D, Purdom S, Casibang M, Zia F, Moody TW (1999) PACAP(6-38) is a PACAP receptor antagonist for breast cancer cells. Breast Cancer Res Treat 56:177–186
Moody TW, Gozes I (2007) Vasoactive intestinal peptide receptors: a molecular target in breast and lung cancer. Cur Pharm Des 13:1099–1104
Jaworski DM (2000) Expression of pituitary adenylate cyclase-activating polypeptide (PACAP) and the PACAP-selective receptor in cultured rat astrocytes, human brain tumors, and in response to acute intracranial injury. Cell Tissue Res 300:219–230
Reubi JC (1995) Neuropeptide receptors in health and disease: the molecular basis for in vivo imaging. J Nucl Med 36:1825–1835
Reubi JC (2000) In vitro evaluation of VIP/PACAP receptors in healthy and diseased human tissues. Clinical implications. Ann N Y Acad Sci 921:1–25
Reubi JC, Laderach U, Waser B et al (2000) Vasoactive intestinal peptide/pituitary adenylate cyclase-activating peptide receptor subtypes in human tumors and their tissues of origin. Cancer Res 60:3105–3112
Zhang K, Aruva MR, Shanthly N, Cardi CA, Patel CA, Rattan S, Cesarone G, Wickstrom E, Thakur ML (2007) Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase activating peptide (PACAP) receptor specific peptide analogues for PET imaging of breast cancer: in vitro/in vivo evaluation. Regul Pept 144:91–100
Zhang K, Aruva MR, Shanthly N, Cardi CA, Rattan S, Patel C, Kim C, McCue P, Wickstrom E, Thakur ML (2008) PET imaging of VPAC1 expression in experimental and spontaneous prostate cancer. J Nucl Med 49:112–121
Truong H, Gomella LG, Thakur ML, Trabulsi EJ (2018) VPAC1-targeted PET/CT scan: improved molecular imaging for the diagnosis of prostate cancer using a novel cell surface antigen. World J Urol 36:719–726
Thakur ML, Zhang K, Berger A, Cavanaugh B, Kim S, Channappa C, Frangos AJ, Wickstrom E, Intenzo CM (2013) VPAC1 receptors for imaging breast cancer: a feasibility study. J Nucl Med 54:1019–1025
Tripathi S, Trabulsi EJ, Gomella L, Kim S, McCue P, Intenzo C, Birbe R, Gandhe A, Kumar P, Thakur M (2016) VPAC1 targeted [64Cu]-TP3805 positron emission tomography imaging of prostate cancer: preliminary evaluation in man. Urology 88:111–118
Tripathi SK, Kumar P, Trabulsi EJ, Kim S, McCue PA, Intenzo C, Berger A, Gomella L, Thakur ML (2017) VPAC1 Targeted [64Cu]-TP3805 kit preparation and its evaluation. Nucl Med Biol 51:55–61
Thakur ML, Aruva MR, Gariepy J, Acton P, Rattan S, Prasad S, Wickstrom E, Alavi A (2004) PET imaging of oncogene overexpression using 64Cu-vasoactive intestinal peptide (VIP) analog: comparison with 99mTc-VIP analog. J Nucl Med 45:1381–1389
Tripathi SK, Kumar P, Jin YY et al (2018) VPAC1 biomarker for imaging triple negative breast cancer [abstract]. J Nucl Med 59:1282P
Allen M, Bjerke M, Edlund H et al (2016) Origin of the U87MG glioma cell line: good news and bad news. Sci Tran Med 8:354re353
Szatmari T, Lumniczky K, Desaknai S, Trajcevski S, Hidvegi EJ, Hamada H, Safrany G (2006) Detailed characterization of the mouse glioma 261 tumor model for experimental glioblastoma therapy. Cancer Sci 97:546–553
Jacobs VL, Valdes PA, Hickey WF, De Leo JA (2011) Current review of in vivo GBM rodent models: emphasis on the CNS-1 tumour model. ASN Neuro 3:e00063
Awde AR, Boisgard R, Theze B, Dubois A, Zheng J, Dolle F, Jacobs AH, Tavitian B, Winkeler A (2013) The translocator protein radioligand [18F]-DPA-714 monitors antitumor effect of erufosine in a rat 9L intracranial glioma model. J Nucl Med 54:2125–2131
Association ABT Brain Tumor Statistics. http://www.abta.org/about-us/news/brain-tumor-statistics/ (accessed 4/4/18)
Huang RY, Neagu MR, Reardon DA, Wen PY (2015) Pitfalls in the neuroimaging of glioblastoma in the era of antiangiogenic and immuno/targeted therapy - detecting illusive disease, defining response. Front Neurol 6:33
Herholz K (2018) PET and MRI in gliomas: progress and perspective. Clin Transl Imaging 6:73–75
Orringer DA, Koo YE, Chen T, Kopelman R, Sagher O, Philbert MA (2009) Small solutions for big problems: the application of nanoparticles to brain tumor diagnosis and therapy. Clin Pharmacol Ther 85:531–534
Blanco VM, Chu Z, LaSance K, Gray BD, Pak KY, Rider T, Greis KD, Qi X (2016) Optical and nuclear imaging of glioblastoma with phosphatidylserine-targeted nanovesicles. Oncotarget 7:32866–32875
Iagaru A, Mosci C, Mittra E, Zaharchuk G, Fischbein N, Harsh G, Li G, Nagpal S, Recht L, Gambhir SS (2015) Glioblastoma multiforme recurrence: an exploratory study of 18F FPPRGD2 PET/CT. Radiology 277:497–506
Kunikowska J, Bartosz K, Leszek K (2018) Glioblastoma multiforme: another potential application for 68Ga-PSMA PET/CT as a guide for targeted therapy. Eur J Nucl Med Mol Imaging 45:886–887
Ozdemir-Kaynak E, Qutub AA, Yesil-Celiktas O (2018) Advances in glioblastoma multiforme treatment: new models for nanoparticle therapy. Front Physi 9:170
Treat LH, McDannold N, Zhang Y, Vykhodtseva N, Hynynen K (2012) Improved anti-tumor effect of liposomal doxorubicin after targeted blood-brain barrier disruption by MRI-guided focused ultrasound in rat glioma. Ultrasound Med Biol 38:1716–1725
Sarkaria JN, Hu LS, Parney IF, Pafundi DH, Brinkmann DH, Laack NN, Giannini C, Burns TC, Kizilbash SH, Laramy JK, Swanson KR, Kaufmann TJ, Brown PD, Agar NYR, Galanis E, Buckner JC, Elmquist WF (2018) Is the blood-brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro-oncology 20:184–191
Ferrari C, Asabella AN, Villano C et al (2015) Copper-64 dichloride as theranostic agent for glioblastoma multiforme: a preclinical study. Biomed Res Int 2015:129764
Valdehita A, Bajo AM, Fernandez-Martinez AB et al (2010) Nuclear localization of vasoactive intestinal peptide (VIP) receptors in human breast cancer. Peptides 31:2035–2045
Acknowledgments
Technical support by Paul A. Jicman and Kim Lee are gratefully acknowledged.
Funding
The research, in part, was supported by NIH/NCI RO1CA157372 (MLT), NIH/NCI 1S10OD012406 (MLT), and NIH/NCI S10RR23709 (MLT).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
MLT holds a granted patent on [64Cu]TP3805. Through TJU, the patent is licensed to NuView Pharmaceuticals. Other authors have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 862 kb)
Rights and permissions
About this article
Cite this article
Tripathi, S.K., Kean, R., Bongiorno, E. et al. Targeting VPAC1 Receptors for Imaging Glioblastoma. Mol Imaging Biol 22, 293–302 (2020). https://doi.org/10.1007/s11307-019-01388-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-019-01388-5