Abstract
Purpose
Quantitative analysis of dopamine transporter (DAT) single-photon emission computed tomography (SPECT) images can enhance diagnostic confidence and improve their potential as a biomarker to monitor the progression of Parkinson’s disease (PD). In the present work, we aim to predict motor outcome from baseline DAT SPECT imaging radiomic features and clinical measures using machine learning techniques.
Procedures
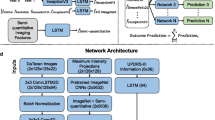
We designed and trained artificial neural networks (ANNs) to analyze the data from 69 patients within the Parkinson’s Progressive Marker Initiative (PPMI) database. The task was to predict the unified PD rating scale (UPDRS) part III motor score in year 4 from 92 imaging features extracted on 12 different regions as well as 6 non-imaging measures at baseline (year 0). We first performed univariate screening (including the adjustment for false discovery) to select 4 regions each having 10 features with significant performance in classifying year 4 motor outcome into two classes of patients (divided by the UPDRS III threshold of 30). The leave-one-out strategy was then applied to train and test the ANNs for individual and combinations of features. The prediction statistics were calculated from 100 rounds of experiments, and the accuracy in appropriate prediction (classification of year 4 outcome) was quantified.
Results
Out of the baseline non-imaging features, only the UPDRS III (at year 0) was predictive, while multiple imaging features depicted significance. The different selected features reached a predictive accuracy of 70 % if used individually. Combining the top imaging features from the selected regions significantly improved the prediction accuracy to 75 % (p < 0.01). The combination of imaging features with the year 0 UPDRS III score also improved the prediction accuracy to 75 %.
Conclusion
This study demonstrated the added predictive value of radiomic features extracted from DAT SPECT images in serving as a biomarker for PD progression tracking.






Similar content being viewed by others
References
Thobois S, Guillouet S, Broussolle E (2001) Contributions of PET and SPECT to the understanding of the pathophysiology of Parkinson’s disease. Neurophysiol Clin 31:321–340
Bajaj N, Hauser RA, Grachev ID (2013) Clinical utility of dopamine transporter single photon emission CT (DaT-SPECT) with 123I ioflupane in diagnosis of parkinsonian syndromes. J Neurol Neurosurg Psychiatry 84:1288–1295
Catafau AM, Tolosa E, Group DCUPSS (2004) Impact of dopamine transporter SPECT using 123I-ioflupane on diagnosis and management of patients with clinically uncertain parkinsonian syndromes. Mov Disord 19:1175–1182
Group PS (2000) A randomized controlled trial comparing pramipexole with levodopa in early Parkinson’s disease: design and methods of the CALM-PD study. Clin Neuropharmacol 23:34–44
Whone AL, Watts RL, Stoessl AJ, Davis M, Reske S, Nahmias C, Lang AE, Rascol O, Ribeiro MJ, Remy P, Poewe WH, Hauser RA, Brooks DJ, REAL-PET Study Group (2003) Slower progression of Parkinson’s disease with ropinirole versus levodopa: the REAL-PET study. Ann Neurol 54:93–101
Fahn S, Parkinson Study Group (2005) Does levodopa slow or hasten the rate of progression of Parkinson’s disease? J Neurol 252(Suppl 4):IV37–IV42
Seibyl J, Jennings D, Tabamo R, Marek K (2005) The role of neuroimaging in the early diagnosis and evaluation of Parkinson’s disease. Minerva Med 96:353–364
Hauser RA, Grosset DG (2012) [123I]FP-CIT (DaTscan) SPECT brain imaging in patients with suspected parkinsonian syndromes. J Neuroimaging 22:225–230
Scherfler C, Schwarz J, Antonini A, Grosset D, Valldeoriola F, Marek K, Oertel W, Tolosa E, Lees AJ, Poewe W (2007) Role of DAT-SPECT in the diagnostic work up of parkinsonism. Mov Disord 22:1229–1238
Ravina B, Marek K, Eberly S, Oakes D, Kurlan R, Ascherio A, Beal F, Beck J, Flagg E, Galpern WR, Harman J, Lang AE, Schwarzschild M, Tanner C, Shoulson I (2012) Dopamine transporter imaging is associated with long-term outcomes in Parkinson’s disease. Mov Disord 27:1392–1397
Parkinson Progression Marker Initiative (2011) The Parkinson progression marker initiative (PPMI). Prog Neurobiol 95:629–635
Rahmim A, Salimpour Y, Jain S, Blinder SAL, Klyuzhin IS, Smith GS, Mari Z, Sossi V (2016) Application of texture analysis to DAT SPECT imaging: relationship to clinical assessments. Neuroimage Clin 12:e1–e9
Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: images are more than pictures, they are data. Radiology 278:563–577
Yip SSF, Liu Y, Parmar C, Li Q, Liu S, Qu F, Ye Z, Gillies RJ, Aerts HJWL (2017) Associations between radiologist-defined semantic and automatically computed radiomic features in non-small cell lung cancer. Sci Rep 7:3519
Schrag A, Siddiqui UF, Anastasiou Z, Weintraub D, Schott JM (2017) Clinical variables and biomarkers in prediction of cognitive impairment in patients with newly diagnosed Parkinson’s disease: a cohort study. Lancet Neurol 16:66–75
Caspell-Garcia C, Simuni T, Tosun-Turgut D, Wu IW, Zhang Y, Nalls M, Singleton A, Shaw LA, Kang JH, Trojanowski JQ, Siderowf A, Coffey C, Lasch S, Aarsland D, Burn D, Chahine LM, Espay AJ, Foster ED, Hawkins KA, Litvan I, Richard I, Weintraub D, the Parkinson’s Progression Markers Initiative (PPMI) (2017) Multiple modality biomarker prediction of cognitive impairment in prospectively followed de novo Parkinson disease. PLoS One 12:e0175674
Nilashi M, Ibrahim O, Ahani A (2016) Accuracy improvement for predicting Parkinson’s disease progression. Sci Rep 6:34181
Emrani S, McGuirk A, Xiao W (2017) Prognosis and diagnosis of Parkinson’s disease using multi-task learning. In: Proceedings of the 23rd ACM SIGKDD international conference on knowledge discovery and data mining. ACM, Halifax, pp 1457–1466
Brown CJ, Miller SP, Booth BG et al (2015) Prediction of motor function in very preterm infants using connectome features and local synthetic instances. In: Navab N., Hornegger J., Wells W., Frangi A. (eds) Medical Image Computing and Computer-Assisted Intervention -- MICCAI 2015. pp 69–76
Ball G, Pazderova L, Chew A, Tusor N, Merchant N, Arichi T, Allsop JM, Cowan FM, Edwards AD, Counsell SJ (2015) Thalamocortical connectivity predicts cognition in children born preterm. Cereb Cortex 25:4310–4318
Guo N, Yen R, El Fakhri G, Li Q (2015) SVM based lung cancer diagnosis using multiple image features in PET/CT. In: 2015 IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC). pp 1–4
Hirschauer TJ, Adeli H, Buford JA (2015) Computer-aided diagnosis of Parkinson’s disease using enhanced probabilistic neural network. J Med Syst 39:179
Prashanth R, Dutta Roy S, Mandal PK, Ghosh S (2016) High-accuracy detection of early Parkinson’s disease through multimodal features and machine learning. Int J Med Inform 90:13–21
Guo N, Guo Z, Shusharina N et al (2017) SVM based radiomics analysis using pre-radiotherapy PET/CT increases the prediction accuracy of radiation pneumonitis. J Nucl Med 58:501
Koch W, Radau PE, Hamann C, Tatsch K (2005) Clinical testing of an optimized software solution for an automated, observer-independent evaluation of dopamine transporter SPECT studies. J Nucl Med 46:1109–1118
Tang X, Oishi K, Faria AV, Hillis AE, Albert MS, Mori S, Miller MI (2013) Bayesian parameter estimation and segmentation in the multi-atlas random orbit model. PLoS One 8:e65591
Evans AC, Collins DL, Mills SR et al (1993) 3D statistical neuroanatomical models from 305 MRI volumes. In: 1993 IEEE conference record nuclear science symposium and medical imaging conference, vol 3. pp 1813–1817
Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM (2002) Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–355
Friston KJ, Ashburner JT, Kiebel SJ et al (2006) Statistical parametric mapping: The analysis of functional brain images. Elsevier, Burlington, pp 49–62
Collignon A, Maes F, Delaere D et al (1995) Automated multi-modality image registration based on information theory. In: Information Processing in Medical Imaging. pp 263–274
Rahmim A, Huang P, Shenkov N, Fotouhi S, Davoodi-Bojd E, Lu L, Mari Z, Soltanian-Zadeh H, Sossi V (2017) Improved prediction of outcome in Parkinson’s disease using radiomics analysis of longitudinal DAT SPECT images. Neuroimage Clin 16:539–544
Altman DG, Bland JM (1995) Statistics notes: the normal distribution. BMJ 310:298
Ghasemi A, Zahediasl S (2012) Normality tests for statistical analysis: a guide for non-statisticians. Int J Endocrinol Metab 10:486–489
Thibault G, Fertil B, Navarro CL et al (2009) Texture indexes and gray level size zone matrix. Application to cell nuclei classification. In: 10th International Conference on Pattern Recognition and Information Processing. pp 140-145
Zeighami Y, Ulla M, Iturria-Medina Y, Dadar M, Zhang Y, Larcher KMH, Fonov V, Evans AC, Collins DL, Dagher A (2015) Network structure of brain atrophy in de novo Parkinson’s disease. Elife 4: e08440
Choi H, Cheon GJ, Kim HJ, Choi SH, Kim YI, Kang KW, Chung JK, Kim EE, Lee DS (2016) Gray matter correlates of dopaminergic degeneration in Parkinson’s disease: a hybrid PET/MR study using 18F-FP-CIT. Hum Brain Mapp 37:1710–1721
Dvornek NC, Ventola P, Pelphrey KA, Duncan JS (2017) Identifying autism from resting-state fMRI using long short-term memory networks. Mach Learn Med Imaging 10541:363-370
Funding
The project was supported by the Michael J. Fox Foundation (Research Grant 2016, ID: 9036.01), including use of data available from the PPMI-a public-private partnership-funded by The Michael J. Fox Foundation for Parkinson’s Research and funding partners (listed at www.ppmi-info.org/fundingpartners). This work was also supported by the National Science Foundation (ECCS 1454552), the Natural Sciences and Engineering Research Council of Canada, and the National Natural Science Foundation of China (grant 61628105).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 2944 kb)
Rights and permissions
About this article
Cite this article
Tang, J., Yang, B., Adams, M.P. et al. Artificial Neural Network–Based Prediction of Outcome in Parkinson’s Disease Patients Using DaTscan SPECT Imaging Features. Mol Imaging Biol 21, 1165–1173 (2019). https://doi.org/10.1007/s11307-019-01334-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-019-01334-5