Abstract
Purpose
To map functional bone marrow (BM) by 2-deoxy-2-[18F]fluoro-d-glucose ([18F]FDG) positron emission tomography (PET) in the vertebral column of lung cancer patients prior to, during, and after treatment. Moreover, to identify radiation- and erlotinib-induced changes in the BM.
Procedures
Twenty-six patients with advanced non-small cell lung cancer, receiving radiotherapy (RT) alone or concomitantly with erlotinib, were examined by [18F]FDG PET before, during, and after treatment. A total of 61 [18F]FDG PET scans were analyzed. Vertebral column BM [18F]FDG standardized uptake value normalized to the liver (SUVBMLR) was used as uptake measure. Wilcoxon signed-rank test was used to assess changes in BM uptake of [18F]FDG between sessions. Effects of erlotinib on the BM activity during and after treatment were assessed using Mann-Whitney U test.
Results
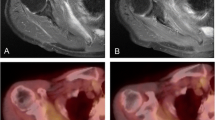
A homogeneous uptake of [18F]FDG was observed within the vertebral column prior to treatment. Mean SUVBMLR (± S.E.M) in the body of thoracic vertebrae receiving a total RT dose of 10 Gy or higher was 0.64 ± 0.01, 0.56 ± 0.01, and 0.59 ± 0.01 at pre-, mid-, and post-therapy, respectively. A significant reduction in the mean SUVBMLR was observed from pre- to both mid- and post-therapy (p < 0.05). Mean SUVBMLR was significantly higher at post-therapy compared to mid-therapy for patients receiving erlotinib in addition to RT (p < 0.05).
Conclusions
RT reduces BM [18F]FDG uptake in the vertebral column, especially in the high-dose region. Concomitant erlotinib may stimulate a recovery in BM [18F]FDG uptake from mid- to post-therapy.
Trial registration: NCT02714530. Registered 10 September 2015.




Similar content being viewed by others
References
Delaney G, Jacob S, Featherstone C, Barton M (2005) The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 104(6):1129–1137
Begg AC, Stewart FA, Vens C (2011) Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer 11(4):239–253
van Baardwijk A, Bosmans G, Boersma L, Wanders S, Dekker A, Dingemans AMC, Bootsma G, Geraedts W, Pitz C, Simons J, Lambin P, de Ruysscher D (2008) Individualized radical radiotherapy of non–small-cell lung cancer based on normal tissue dose constraints: a feasibility study. Int J Radiat Oncol Biol Phys 71(5):1394–1401
Berg JM, Tymoczko JL, Stryer L (2006) Each organ has a unique metabolic profile. In: Biochemistry. W.H.Freeman & Co Ltd, New York, pp 851–854
Fan C, Hernandez-Pampaloni M, Houseni M, Chamroonrat W, Basu S, Kumar R, Dadparvar S, Torigian DA, Alavi A (2007) Age-related changes in the metabolic activity and distribution of the red marrow as demonstrated by 2-deoxy-2-[F-18]fluoro-D-glucose-positron emission tomography. Mol Imaging Biol 9(5):300–307
Dudek A, Mahaseth H (2005) Circulating angiogenic cytokines in patients with advanced non-small cell lung cancer: correlation with treatment response and survival. Cancer Invest 23(3):193–200
Colasante A, Mascetra N, Brunetti M et al (1997) Transforming growth factor beta 1, interleukin-8 and interleukin-1, in non-small-cell lung tumors. Am J Respir Crit Care Med 156(3):968–973
Patchen ML, MacVittie TJ, Williams JL, Schwartz GN, Souza LM (1991) Administration of interleukin-6 stimulates multilineage hematopoiesis and accelerates recovery from radiation-induced hematopoietic depression. Blood 77(3):472–480
Ramadori G, Van Damme J, Rieder H, Meyer zum Büschenfelde KH (1988) Interleukin 6, the third mediator of acute-phase reaction, modulates hepatic protein synthesis in human and mouse. Comparison with interleukin 1 p and tumor necrosis factor-a. Eur. J. Immuno 18:1259–1264
Deek MP, Benenati B, Kim S, Chen T, Ahmed I, Zou W, Aisner J, Jabbour SK (2016) Thoracic vertebral body irradiation contributes to acute hematologic toxicity during chemoradiation therapy for non-small cell lung cancer. Int J Radiat Oncol Biol Phys 94(1):147–154
Fairchild A, Harris K, Barnes E, Wong R, Lutz S, Bezjak A, Cheung P, Chow E (2008) Palliative thoracic radiotherapy for lung cancer: a systematic review. J Clin Oncol 26(24):4001–4011
Chen X, Liu Y, Røe OD, Qian Y, Guo R, Zhu L, Yin Y, Shu Y (2013) Gefitinib or erlotinib as maintenance therapy in patients with advanced stage non-small cell lung cancer: a systematic review. PLoS One 8(3):e59314
Hicks RJ, Kalff V, MacManus MP, Ware RE, Hogg A, McKenzie A, Matthews JP, Ball DL (2001) 18F-FDG PET provides high-impact and powerful prognostic stratification in staging newly diagnosed non-small cell lung cancer. J Nucl Med 42(11):1596–1604
Jiménez-Bonilla JF, Quirce R, Martínez-Rodríguez I, Banzo I, Rubio-Vassallo AS, del Castillo-Matos R, Ortega-Nava F, Martínez-Amador N, Ibáñez-Bravo S, Carril JM (2013) Diagnosis of recurrence and assessment of post-recurrence survival in patients with extracranial non-small cell lung cancer evaluated by 18F-FDG PET/CT. Lung Cancer 81(1):71–76
Gordon BA, Flanagan FL, Dehdashti F (1997) Whole-body positron emission tomography: normal variations, pitfalls, and technical considerations. AJR Am J Roentgenol 169(6):1675–1680
Lee JW, Seo KH, Kim E-S, Lee SM (2016) The role of 18F-fluorodeoxyglucose uptake of bone marrow on PET/CT in predicting clinical outcomes in non-small cell lung cancer patients treated with chemoradiotherapy. Eur Radiol 27(5):1912–1921
Rose BS, Liang Y, Lau SK, Jensen LG, Yashar CM, Hoh CK, Mell LK (2012) Correlation between radiation dose to 18F-FDG-PET defined active bone marrow subregions and acute hematologic toxicity in cervical cancer patients treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys 83(4):1185–1191
Liang Y, Bydder M, Yashar CM, Rose BS, Cornell M, Hoh CK, Lawson JD, Einck J, Saenz C, Fanta P, Mundt AJ, Bydder GM, Mell LK (2013) Prospective study of functional bone marrow-sparing intensity modulated radiation therapy with concurrent chemotherapy for pelvic malignancies. Int J Radiat Oncol Biol Phys 85(2):406–414
Franco P, Arcadipane F, Ragona R, Lesca A, Gallio E, Mistrangelo M, Cassoni P, Arena V, Bustreo S, Faletti R, Rondi N, Morino M, Ricardi U (2016) Dose to specific subregions of pelvic bone marrow defined with FDG-PET as a predictor of hematologic nadirs during concomitant chemoradiation in anal cancer patients. Med Oncol 33(7):72
Rose BS, Jee KW, Niemierko A, Murphy JE, Blaszkowsky LS, Allen JN, Lee LK, Wang Y, Drapek LC, Hong TS, Wo JY (2016) Irradiation of FDG-PET-defined active bone marrow subregions and acute hematologic toxicity in anal cancer patients undergoing chemoradiation. Int J Radiat Oncol Biol Phys 94(4):747–754
Yagi M, Froelich J, Arentsen L, Shanley R, Ghebre R, Yee D, Hui S (2015) Longitudinal FDG-PET revealed regional functional heterogeneity of bone marrow, site-dependent response to treatment and correlation with hematological parameters. J Cancer 6(6):531–537
Noticewala SS, Li N, Williamson CW et al (2017) Longitudinal changes in active bone marrow for cervical cancer patients treated with concurrent chemoradiation therapy. Int J Radiat Oncol Biol Phys 97(4):797–805
Hayman JA, Callahan JW, Herschtal A, Everitt S, Binns DS, Hicks RJ, Mac Manus M (2011) Distribution of proliferating bone marrow in adult cancer patients determined using FLT-PET imaging. Int J Radiat Oncol Biol Phys 79(3):847–852
Inoue K, Goto R, Okada K, Kinomura S, Fukuda H (2009) A bone marrow F-18 FDG uptake exceeding the liver uptake may indicate bone marrow hyperactivity. Ann Nucl Med 23(7):643–649
Abravan A, Eide HA, Knudtsen I, Løndalen A, Helland Å, Malinen E (2017) Assessment of pulmonary 18F-FDG-PET uptake and cytokine profiles in non-small cell lung cancer patients treated with radiotherapy and erlotinib. Clin Transl Radiat Oncol 4:57–63
Eide HA, Halvorsen AR, Sandhu V, et al. (2016) Non-small cell lung cancer is characterised by a distinct inflammatory signature in serum compared with chronic obstructive pulmonary disease. Clin Trans Immunol 5(11):e109. https://doi.org/10.1038/cti.2016.65
Mauch P, Constine L, Greenberger J, Knospe W, Sullivan J, Liesveld JL, Deeg HJ (1995) Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. Int J Radiat Oncol Biol Phys 31(5):1319–1339
Hall EJ, Giaccia AJ (2006) Dose–response relationships for model normal tissues. In: Mitchell CW (ed) Radiobiology for the radiologist. Lippincott Williams & Wilkins, Philadelphia, pp 303–326
Formenti SC, Demaria S (2013) Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst 105(4):256–265
Heylmann D, Rödel F, Kindler T, Kaina B (2014) Radiation sensitivity of human and murine peripheral blood lymphocytes, stem and progenitor cells. Biochim Biophys Acta 1846(1):121–129
Higashi T, Fisher SJ, Brown RS, Nakada K, Walter GL, Wahl RL (2000) Evaluation of the early effect of local irradiation on normal rodent bone marrow metabolism using FDG: preclinical PET studies. J Nucl Med 41(12):2026–2035
Ryan MA, Nattamai KJ, Xing E, Schleimer D, Daria D, Sengupta A, Köhler A, Liu W, Gunzer M, Jansen M, Ratner N, le Cras TD, Waterstrat A, van Zant G, Cancelas JA, Zheng Y, Geiger H (2010) Pharmacological inhibition of EGFR signaling enhances G-CSF–induced hematopoietic stem cell mobilization. Nat Med 16:1141–1146
Doan PL, Himburg HA, Helms K, Russell JL, Fixsen E, Quarmyne M, Harris JR, Deoliviera D, Sullivan JM, Chao NJ, Kirsch DG, Chute JP (2013) Epidermal growth factor regulates hematopoietic regeneration following radiation injury. Nat Med 19(3):295–304
Bernad A, Kopf M, Kulbacki R, Weich N, Koehler G, Gutierrez-Ramos JC Interleukin-6 is required in vivo for the regulation of stem cells and committed progenitors of the hematopoietic system. Immunity 1(9):725–731
Fletcher EVM, Love-Homan L, Sobhakumari A, et al. (2013) Epidermal growth factor receptor inhibition induces pro-inflammatory cytokines via NOX4 in head and neck cancer cells. Mol Cancer Res
Acknowledgements
We gratefully thank Ingerid Skjei Knudtsen, University of Oslo, Norway for her assistance in setting up the PET/CT protocol and data collection. This study was partly funded by the faculty of Mathematics and Natural Sciences, University of Oslo, Norway and was supported by the Norwegian Cancer Society and The regional health authorities in South East Norway.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the Regional Committee for Medical and Health Research Ethics. A written informed consent was received from all patients.
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 367 kb)
Rights and permissions
About this article
Cite this article
Abravan, A., Eide, H.A., Løndalen, A.M. et al. Mapping Bone Marrow Response in the Vertebral Column by Positron Emission Tomography Following Radiotherapy and Erlotinib Therapy of Lung Cancer. Mol Imaging Biol 21, 391–398 (2019). https://doi.org/10.1007/s11307-018-1226-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-018-1226-7