Abstract
Purpose
We aimed to develop a radiolabeled peptide probe for the imaging of hypoxia-inducible factor-1 (HIF-1)-active tumors.
Procedures
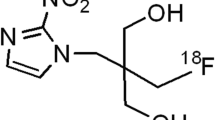
We synthesized the peptide probes that contain or lack an essential sequence of the oxygen-dependent degradation of HIF-1α in proteasomes (123/125I-DKOP30 or 125I-mDKOP, respectively). The degradation of probes was evaluated in vitro using cell lysates containing proteasomes. In vivo biodistribution study, planar imaging, autoradiography, and comparison between probe accumulation and HIF-1 transcriptional activity were also performed.
Results
The 125I-DKOP30 underwent degradation in a proteasome-dependent manner, while 125I-mDKOP was not degraded. Biodistribution analysis showed 125I-DKOP30 accumulation in tumors. The tumors were clearly visualized by in vivo imaging, and intratumoral distribution of 125I-DKOP30 coincided with the HIF-1α-positive hypoxic regions. Tumoral accumulation of 125I-DKOP30 was significantly correlated with HIF-1-dependent luciferase bioluminescence, while that of 125I-mDKOP was not.
Conclusion
123I-DKOP30 is a useful peptide probe for the imaging of HIF-1-active tumors.





Similar content being viewed by others
References
Harada H (2011) How can we overcome tumor hypoxia in radiation therapy? J Radiat Res 52:545–556
Ogawa K, Chiba I, Morioka T et al (2011) Clinical significance of HIF-1alpha expression in patients with esophageal cancer treated with concurrent chemoradiotherapy. Anticancer Res 31:2351–2359
Rohwer N, Cramer T (2011) Hypoxia-mediated drug resistance: novel insights on the functional interaction of HIFs and cell death pathways. Drug Resist Updat 14:191–201
Keith B, Johnson RS, Simon MC (2012) HIF1alpha and HIF2alpha: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer 12:9–22
Lu X, Kang Y (2010) Hypoxia and hypoxia-inducible factors: master regulators of metastasis. Clin Cancer Res 16:5928–5935
Semenza GL (2011) Oxygen sensing, homeostasis, and disease. N Engl J Med 365:537–547
Semenza GL (2012) Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci 33:207–214
Semenza GL (2012) Hypoxia-inducible factors in physiology and medicine. Cell 148:399–408
Jiang BH, Semenza GL, Bauer C, Marti HH (1996) Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol 271:C1172–C1180
Stroka DM, Burkhardt T, Desbaillets I et al (2001) HIF-1 is expressed in normoxic tissue and displays an organ-specific regulation under systemic hypoxia. FASEB J 15:2445–2453
Brahimi-Horn MC, Pouyssegur J (2007) Oxygen, a source of life and stress. FEBS Lett 581:3582–3591
Kizaka-Kondoh S, Tanaka S, Harada H, Hiraoka M (2009) The HIF-1-active microenvironment: an environmental target for cancer therapy. Adv Drug Deliv Rev 61:623–632
Kudo T, Ueda M, Kuge Y et al (2009) Imaging of HIF-1-active tumor hypoxia using a protein effectively delivered to and specifically stabilized in HIF-1-active tumor cells. J Nucl Med 50:942–949
Ueda M, Kudo T, Mutou Y et al (2011) Evaluation of [125I]IPOS as a molecular imaging probe for hypoxia-inducible factor-1-active regions in a tumor: comparison among single-photon emission computed tomography/X-ray computed tomography imaging, autoradiography, and immunohistochemistry. Cancer Sci 102:2090–2096
Fujii H, Yamaguchi M, Inoue K et al (2012) In vivo visualization of heterogeneous intratumoral distribution of hypoxia-inducible factor-1alpha activity by the fusion of high-resolution SPECT and morphological imaging tests. J Biomed Biotechnol 2012:262741
Ueda M (2012) Development of a method for high-contrasted nuclear medical imaging of hypoxia-inducible factor-1-active tumor by using a pretargeting approach. Yakugaku Zasshi 132:595–600
Khawli LA, van den Abbeele AD, Kassis AI (1992) N-(m-[125I]iodophenyl)maleimide: an agent for high yield radiolabeling of antibodies. Int J Rad Appl Instrum B 19:289–295
Driscoll J, Goldberg AL (1990) The proteasome (multicatalytic protease) is a component of the 1500-kDa proteolytic complex which degrades ubiquitin-conjugated proteins. J Biol Chem 265:4789–4792
Kuchimaru T, Kadonosono T, Tanaka S et al (2010) In vivo imaging of HIF-active tumors by an oxygen-dependent degradation protein probe with an interchangeable labeling system. PLoS One 5:e15736
Ueda M, Kudo T, Kuge Y et al (2010) Rapid detection of hypoxia-inducible factor-1-active tumours: pretargeted imaging with a protein degrading in a mechanism similar to hypoxia-inducible factor-1alpha. Eur J Nucl Med Mol Imaging 37:1566–1574
Kudo T, Ueda M, Konishi H et al (2011) PET imaging of hypoxia-inducible factor-1-active tumor cells with pretargeted oxygen-dependent degradable streptavidin and a novel 18F-labeled biotin derivative. Mol Imaging Biol 13:1003–1010
Baudelet C, Gallez B (2004) Effect of anesthesia on the signal intensity in tumors using BOLD-MRI: comparison with flow measurements by laser Doppler flowmetry and oxygen measurements by luminescence-based probes. Magn Reson Imaging 22:905–912
Semenza GL (2001) HIF-1, O2, and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell 107:1–3
Jaakkola P, Mole DR, Tian YM et al (2001) Targeting of HIF-alpha to the von Hippel–Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292:468–472
Paltoglou S, Roberts BJ (2007) HIF-1alpha and EPAS ubiquitination mediated by the VHL tumour suppressor involves flexibility in the ubiquitination mechanism, similar to other RING E3 ligases. Oncogene 26:604–609
Harada H, Hiraoka M, Kizaka-Kondoh S (2002) Antitumor effect of TAT-oxygen-dependent degradation-caspase-3 fusion protein specifically stabilized and activated in hypoxic tumor cells. Cancer Res 62:2013–2018
Nakase I, Konishi Y, Ueda M, Saji H, Futaki S (2012) Accumulation of arginine-rich cell-penetrating peptides in tumors and the potential for anticancer drug delivery in vivo. J Control Release 159:181–188
Dales JP, Garcia S, Meunier-Carpentier S et al (2005) Overexpression of hypoxia-inducible factor HIF-1alpha predicts early relapse in breast cancer: retrospective study in a series of 745 patients. Int J Cancer 116:734–739
Park S, Ha SY, Cho HY et al (2011) Prognostic implications of hypoxia-inducible factor-1alpha in epidermal growth factor receptor-negative non-small cell lung cancer. Lung Cancer 72:100–107
Eckert AW, Lautner MH, Schutze A et al (2011) Coexpression of hypoxia-inducible factor-1alpha and glucose transporter-1 is associated with poor prognosis in oral squamous cell carcinoma patients. Histopathology 58:1136–1147
Kersemans V, Kersemans K, Cornelissen B (2008) Cell penetrating peptides for in vivo molecular imaging applications. Curr Pharm Des 14:2415–2447
Koh MY, Powis G (2012) Passing the baton: the HIF switch. Trends Biochem Sci 37:364–372
Acknowledgments
The authors would like to thank Nihon Medi-Physics Co. Ltd. for providing ammonium [123I]iodide. This work was supported in part by the Research and Development Project on Molecular Probes for Detection of Biological Features on Cancer of the New Energy and Industrial Technology Development Organization (NEDO), Japan, and a Grant-in-Aid for Young Scientists (B) (KAKENHI Grant Number 23791412) from the Japan Society for the Promotion of Science.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ueda, M., Ogawa, K., Miyano, A. et al. Development of an Oxygen-Sensitive Degradable Peptide Probe for the Imaging of Hypoxia-Inducible Factor-1-Active Regions in Tumors. Mol Imaging Biol 15, 713–721 (2013). https://doi.org/10.1007/s11307-013-0647-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-013-0647-6