Abstract
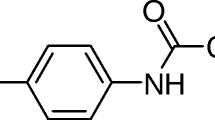
The purpose of this piece of work is to study the process of adsorption of paracetamol on activated carbon, silica and alumina and their degradation using UV radiation. The results demonstrate a higher adsorption of paracetamol on alumina and activated carbon, while a minor value was observed in the case of silica. The H-bonding and π-stacking interactions between paracetamol and supports can be explained by the variation in the adsorption capacity values. When the paracetamol adsorbed was irradiated with two different UV irradiance values (59.78 mW cm−2 and 119.56 mW cm−2) for 120 min, the higher degradation percentage was observed on activated carbon with a value of 79%. In the case of alumina and silica, the maximum percentages obtained were 65% and 77%, respectively. The incorporation of H2O2 in the reaction medium increases the rate of degradation, mainly at higher irradiance, reaching the maximum values in less time.



Similar content being viewed by others
References
Abdel-Wahaba, A., Al-Shirbinib, A., Mohameda, O., & Nasra, O. (2017). Photocatalytic degradation of paracetamol over magnetic flower-like TiO2/Fe2O3 core-shell nanostructures. Journal of Photochemistry and Photobiology A: Chemistry, 347, 186–198.
Andrieux, D., Jestin, J., Kervarec, N., Pichon, R., Privat, M., & Olier, R. (2004). Adsorption mechanism of substituted pyridines on silica suspensions: an NMR study. Langmuir, 20, 10591–10598.
Desale, A., Kamble, S., & Deosarkar, M. (2013). Photocatalytic degradation of paracetamol using degussa TiO2 photocatalyst. International Journal of Chemical & Physical Sciences, 2, 140–148.
Eda, G., Lin, Y. Y., Mattevi, C., Yamaguchi, H., Chen, H. A., Chen, I. S., Chen, C. W., & Chhowalla, M. (2010) Blue photoluminescence from chemically derived graphene oxide. Advanced Materials, 22, 505–509.
Ferreira, R. C., De Lima, H. H. C., Cândido, A. A., Couto, O. M., Arroyo, P. A., De Carvalho, K. Q., Gauze, G. F., & Barros, M. A. (2015a). Adsorption of paracetamol using activated carbon of dende and babassu coconut mesocarp. World Academy of Science, Engineering and Technology International Journal of Biotechnology and Bioengineering, 9, 717–722.
Ferreira, R. C., Couto, O. M., De Carvalho, K. Q., & Arroyo, B. M. A. (2015b). Effect of solution pH on the removal of paracetamol by activated carbon of dende coconut mesocarp. Chemical and Biochemical Engineering Quarterly, 29, 47–53.
Fioressi, S., & Arce, R. (2005). Photochemical transformations of benzo [e] pyrene in solution and adsorbed on silica gel and alumina surfaces. Environmental Science & Technology, 39, 3646–3655.
Ghafoori, S., Mowla, A., Jahani, R., Mehrvar, M., & Chan, P. (2015). Sonophotolytic degradation of synthetic pharmaceutical wastewater: statistical experimental design and modelling. Journal of Environmental Management, 150, 128–137.
Gogate, P., & Pandit, A. (2004). A review of imperative technologies for wastewater treatment I: oxidation technologies at ambient conditions. Advances in Environmental Research, 49(8), 501–551.
Haque, M., & Muneer, M. (2007). Photodegradation of norfloxacin in aqueous suspensions of titanium dioxide. Journal of Hazardous Materials, 145, 51–57.
Harris, M., Karper, E., Stacks, G., Hoffman, D., DeNiro, R., Cruz, P., et al. (2001). Writing labs and the Hollywood connection. Journal of Film Writing, 44(3), 213–245.
Jallouli, N., Elghniji, K., Trabel, H., & Ksibi, M. (2017). Photocatalytic degradation of paracetamol on TiO2 nanoparticles and TiO2/cellulosic fiber under UV and sunlight irradiation. Arabian Journal of Chemistry, 10, S3640–S3645.
Kanakaraju, D., Glass, B., & Oelgemöller, M. (2018). Advanced oxidation process mediated removal of pharmaceuticals from water: a review. Journal of Environmental Management, 219, 189–207.
Karunakaran, C., Dhanalakshmi, R., Manikandan, G., & Gomathisankar, P. (2011). Photodegradation of carboxylic acids on Al2O3 and SiO2 nanoparticles. Indian Journal of Chemistry, 50, 163–170.
Klosek, S., & Raftery, D. (2001). Visible light driven V-doped TiO2 photocatalyst and its photo-oxidation of ethanol. Journal of Physical Chemistry B, 105, 2815–2819.
Lladó, J., Lao-Luque, C., Ruiz, B., Fuente, E., Solé-Sardans, M., & Dorado, A. (2015). Role of activated carbon properties in atrazine and paracetamol adsorption equilibrium and kinetics. Process Safety and Environmental Protection, 95, 51–59.
Lorphensri, O., Intravijit, J., Sabatini, A. D., Kibbey, T., Osathaphan, K., & Saiwan, C. (2006). Sorption of acetaminophen, 17 α-ethynyl estradiol, nalidixic acid, and norfloxacin to silica, alumina, and a hydrophobic médium. Water Research, 40, 1481–1491.
Luna, M., Veciana, M., Su, C., & Lu, M. (2012). Acetaminophen degradation by electro-Fenton and photoelectro-Fenton using a double cathode electrochemical cell. Journal of Hazardous Materials, 217, 200–207.
Mameri, Y., Debbache, N., Benacherine, M., Seraghni, N., & Sehili, T. (2016). Heterogeneous photodegradation of paracetamol using goethite/H2O2 and goethite/oxalic acid systems under artificial and natural light. Journal of Photochemistry and Photobiology A: Chemistry, 315, 129–137.
Matus, C., Camú, E., Villarroel, M., Ojeda, J., & Baeza, P. (2016). Study of the removal of 4–nitrophenol from aqueous media by adsorption on different materials. Journal of the Chilean Chemical Society, 61, 2898–2902.
Moreno-Castilla, C. (2004). Adsorption of organic molecules from aqueous solutions on carbon materials. Carbon, 42, 83–94.
Peralta, C., Camú, E., Bassi, R., Villarroel, M., Ojeda, J., & Baeza, P. (2016). Denitrogenation by adsorption of pyridine on Ni/support adsorbents. Journal of the Chilean Chemical Society, 61, 3211–3213.
Quesada, I., Julcour, C., Jáuregui, U., Wilhelm, A., & Delmas, H. (2009). Sonolysis of levodopa and paracetamol in aqueous solutions. Ultrasonics Sonochemistry, 16, 610–616.
Sellaoui, L., Lima, E., Dotto, G., & Lamine, A. (2017). Adsorption of amoxicillin and paracetamol on modified activated carbons: equilibrium and positional entropy studies. Journal of Molecular Liquids, 234, 375–381.
Siré, I., Garrido, J., Rodriguez, R., Cabot, P., Centellas, F., Arias, C., & Brillas, E. (2006). Electrochemical degradation of paracetamol from water by catalytic action of Fe2+, Cu2+, and UVA light on electrogenerated hydrogen peroxide. Journal of the Electrochemical Society, 153, D1–D9.
Sophia, C., & Lima, E. (2018). Removal of emerging contaminants from the environment by adsorption. Ecotoxicology and Environmental Safety, 161, 57–63.
Terzyk, A. (2004). Molecular properties and intermolecular forces—factors balancing the effect of carbon surface chemistry in adsorption of organics from dilute aqueous solutions. Journal of Colloid and Interface Science, 275, 9–29.
Thi, V., & Lee, B. (2017). Effective photocatalytic degradation of paracetamol using La-doped ZnO photocatalyst under visible light irradiation. Materials Research Bulletin, 96, 171–182.
Tortet, L., Ligner, E., Blanluet, W., Noguez, P., Marichal, C., Schäf, O., & Paillaud, J. (2017). Adsorptive elimination of paracetamol from physiological solutions: interaction with MFI-type zeolite. Microporous and Mesoporous Materials, 252, 188–196.
Velo-Gala, I., López-Peñalver, J., Sánchez-Polo, M., & Rivera-Utrilla, J. (2017). Applied Catalysis B: Environmental (Vol. 207, pp. 412–423).
Villaescusa, I., Fiol, N., Poch, J., Bianchi, A., & Bazzicalupi, C. (2011). Mechanism of paracetamol removal by vegetable wastes: the contribution of π–π interactions, hydrogen bonding and hydrophobic effect. Desalination, 270, 135–142.
Vogna, D., Marotta, R., Napolitano, A., & D’Ischia, M. (2002). Advanced oxidation chemistry of paracetamol. UV/H2O2-induced hydroxylation/degradation pathways and 15N-aided inventory of nitrogenous breakdown products. Journal of Organic Chemistry, 67, 6143–6151.
Wu, Y., Qi, H., Shi, C., Ma, R., Liu, S., & Huang, Z. (2017). Preparation and adsorption behaviors of sodium alginate-based adsorbent-immobilized β- cyclodextrin and graphene oxide. The Royal Society of Chemistry, 7, 31549–31557.
Yang, L., Yu, L., & Ray, M. (2008). Degradation of paracetamol in aqueous solutions by TiO2 photocatalysis. Water Research, 50, 343488.
Yang, L., Yu, L., & Ray, M. (2009). Photocatalytic oxidation of paracetamol: dominant reactants, intermediates, and reaction mechanisms. Environmental Science and Technology, 2009(43), 460–465.
Zhang, X., Wu, F., Wu, X., Chen, P., & Deng, N. (2008). Photodegradation of acetaminophen in TiO2 suspended solution. Journal of Hazardous Materials, 157, 300–307.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Baeza, P., Aballay, P., Matus, C. et al. Degradation of Paracetamol Adsorbed on Inorganic Supports Under UV Irradiation. Water Air Soil Pollut 230, 34 (2019). https://doi.org/10.1007/s11270-019-4095-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-019-4095-z