Abstract
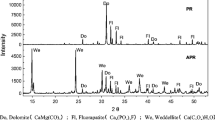
The first uranium extraction mine of Brazil, nowadays found in decommissioning phase, has caused several negative environmental impacts in its area, as a result of mining, treatment, and beneficiation processes. The generation of acid mine drainage in waste rock pile 4 (WR-4) is one of the negative impacts with the most critical situation. The acidic water, product of this drainage, presents heavy metals and radioactive elements and it may be infiltrated by the basis of the impoundment basin, where this water is collected for treatment. The objective of this study was to investigate a typical tropical soil, located in the area of Ores Treatment Unit of Caldas in the southwestern state of Minas Gerais, Brazil, in order to use it as a mineral liner for a retention basin to minimize leakage of acidic water through the foundation of a containment dam. In this way, geotechnical, chemical, and mineralogical tests were performed in order to characterize a soil sample collected in the area. In addition, adsorption tests were conducted with solutions of aluminum (Al), manganese (Mn), and iron (Fe), and with and without adjustment of the initial pH (pHto) of the solutions. The results indicated a well-weathered soil composed of kaolinite, gibbsite, and iron oxides. The adsorption tests showed different behaviors for Al, Mn, and Fe considering or not the adjustment of the pHto. Aluminum showed low adsorption by soil; because of this, only the adsorption isotherms of Mn and Fe for test with adjustment of the pHt0 were determinate. The coefficient of distribution (K D) of Mn was 0.0364 L g−1 and Fe 0.0281 L g−1. As for the retardation factor (R d), its values ranged from 81 to 91 for Mn and from 61 to 79 for Fe, considering different behaviors of the adsorption isotherm models.







Similar content being viewed by others
References
ASTM D 422-63. (2007). Standard test method for particle-size analysis of soils. West Conshohocken: American Society for Testing and Materials.
ASTM D 4318-10. (2010). Standard test method for liquid limit, plastic limit and plasticity index of soils. West Conshohocken: American Society for Testing and Materials.
ASTM D 698-12. (2012). Standard test methods for laboratory compaction characteristics of soil using standard effort (12 400 ft-lbf/ft 3 (600 kN-m/m 3 )). West Conshohocken: American Society for Testing and Materials.
ASTM D 854-14. (2014). Standard test methods for specific gravity of soil solids by water pycnometer. West Conshohocken: American Society for Testing and Materials.
Azevedo, I. C. D., Nascentes, C. R., Matos, A. T., & Azevedo, R. F. (2005). Determinação de parâmetros de transporte de metais pesados em Latossolo compactado. Revista Brasileira de Engenharia Agrícola e Ambientale, 9, 623–630.
Camargo, O. A., Moniz, A. C., Jorge, J. A., & Valadares, J. M. A. S. (2009). Métodos de Análises Químicas, Físicas e Mineralógicas do Solo do Instituto Agronômico de Campinas. Boletim Técnico 106. Campinas: Instituto Agronômico de Campinas.
Campos, M. B., de Azevedo, H., Nascimento, M. R. L., Roque, C. V., & Rodgher, S. (2011). Environmental assessment of water from a uranium mine (Caldas, Minas Gerais State, Brazil) in a decommissioning operation. Environmental Earth Science, 62, 857–863.
Casagrande, A. (1948). Classification and identification of soils. Transactions. Proceedings of the American Society of Civil Engineers, 113, 901–991.
Cipriani, M. (2002). Mitigação dos Impactos Sociais e Ambientais Decorrentes do Fechamento Definitivo de Minas de Urânio. Tese (Doutor em Ciências). Universidade Estadual de Campinas, Campinas: Unicamp.
CONAMA, Conselho Nacional do Meio Ambiente. (2005). Resolução n◦ 357. Brasília: Ministério do Meio Ambiente. Available: www.mma.gov.br/port/conama/index.cfm.
Diz, H. R., Novak, J. T., & Rimstidt, J. D. (1999). Iron precipitation kinetics in synthetic acid mine drainage. Mine Water and the Environment, 18, 1–14.
Fernandes, H. M., Franklin, M. R., & Veiga, L. H. (1998). Acid rock drainage and radiological environmental impacts. A study case of the Uranium mining and milling facilities at Poços de Caldas. Waste Management, 18, 169–181.
Franklin, M. R. (2007). Modelagem Numérica do Escoamento Hidrológico e dos Processos Geoquímicos Aplicados à Previsão De Drenagem Ácida em uma Pilha de Estéril da Mina de Urânio de Poços de Caldas – MG. Tese (Doutorado em Engenharia Civil). Universidade do Rio de Janeiro, Rio de Janeiro: COPPE.
Freeze, R.A. & Cherry, J. A. (1979). Groundwater. Prentice-Hall 500 p.
Garda, G. M. (1990). A Alteração Hidrotermal no Contexto da Evolução Geológica do Maciço Alcalino de Poços de Caldas, MG-SP. Dissertação (Mestrado em Geociências). Universidade de São Paulo, São Paulo: USP.
Krauskopf, K. B. (1979). Introduction to geochemistry (2nd ed.). New York: McGraw-Hill.
Ladeira, A. C. Q., & Gonçalves, C. R. (2007). Influence of anionic species on uranium separation from acid mine water using strong base resins. Journal of Hazardous Materials, 48, 499–504.
Limousin, G., Gaudet, J. P., Charlet, L., Szenknect, S., Barthès, V., & Krimissa, M. (2007). Sorption isotherms: a review on physical bases, modeling and measurement. Applied Geochemistry, 22, 249–275.
McCafferty, E. (2010). Introduction to corrosion science. New York: Springer.
Motsi, T., Rowson, N. A., & Simmons, M. J. H. (2009). Adsorption of heavy metals from acid mine drainage by natural zeolite. International Journal of Mineral Processing, 92, 42–48.
Nogami, J. S., & Villibor, D. F. (1995). Paving low cost lateritic soils. São Paulo: Villibor.
Roy, W.R., Kaprac, I.G., Chou, S.F.J., & Griffin, R.A. (1992). Batch type procedures for estimating soil adsorption of chemicals. EPA 530/SW-87-006. Cincinnati, EUA.
Shamsguddin, J., & Anda, M. (2008). Charge properties of soils in Malaysia dominated by kaolinite, gibbsite, goethite and hematite. Bulletin Geological Society of Malaysia, 54, 27–31.
Silva, A. M., Cruz, F. L. D., Lima, R. M. F., Teixeira, M. C., & Leão, V. A. (2010). Manganese and limestone interactions during mine water treatment. Journal of Hazardous Materials, 181, 514–520.
Singh, G., & Rawat, N. S. (1985). Removal of trace elements from acid mine drainage. International Journal of Mine Water, 4, 17–23.
Sposito, G., (2008). The Chemical of the Soils. (2nd ed). New York: Oxford University Press.
Uehara, G., & Gillman, G. P. (1981). The mineralogy, chemistry, and physics of tropical soils with variable charge clays. Boulder: Westview Press.
USDA, United States Department of Agriculture (1998). Soil quality indicators: pH. Soil quality information sheet. USDA Natural Resources Conservation Service.
Vazquez, O., Pu, X., Monnell, J. D., & Neufeld, R. D. (2010). Release of aluminum from clays in an acid rock drainage. Environment Mine Water and the Environment, 29, 270–276.
Acknowledgments
The authors would like to thank the National Nuclear Energy Commission (CNEN) and the Nuclear Industries of Brazil SA (INB) for the support given to the research, and the National Research Council (CNPq) for granting of scholarship for masters (process number 300789/2011-4).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miguel, M.G., Barreto, R.P. & Pereira, S.Y. Analysis of Aluminum, Manganese, and Iron Adsorption for the Design of a Liner for Retention of the Acid Mining Drainage. Water Air Soil Pollut 226, 67 (2015). https://doi.org/10.1007/s11270-015-2297-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-015-2297-6