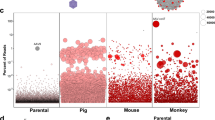
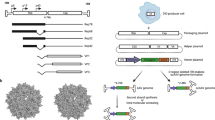
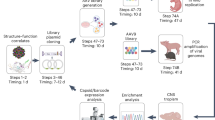
Abstract
During the past five decades, it has become evident that Adeno-associated virus (AAV) represents one of the most potent, most versatile, and thus most auspicious platforms available for gene delivery into cells, animals and, ultimately, humans. Particularly attractive is the ease with which the viral capsid—the major determinant of virus–host interaction including cell specificity and antibody recognition—can be modified and optimized at will. This has motivated countless researchers to develop high-throughput technologies in which genetically engineered AAV capsid libraries are subjected to a vastly hastened emulation of natural evolution, with the aim to enrich novel synthetic AAV capsids displaying superior features for clinical application. While the power and potential of these forward genetics approaches is undisputed, they are also inherently challenging as success depends on a combination of library quality, fidelity, and complexity. Here, we will describe and discuss two original, very exciting strategies that have emerged over the last three years and that promise to alleviate at least some of these concerns, namely, (i) a reverse genetics approach termed “ancestral AAV sequence reconstruction,” and (ii) AAV genome barcoding as a technology that can advance both, forward and reverse genetics stratagems. Notably, despite the conceptual differences of these two technologies, they pursue the same goal which is tailored acceleration of AAV evolution and thus winning the race for the next-generation AAV vectors for clinical use.


Similar content being viewed by others
References
R.W. Atchison, B.C. Casto, W.M. Hammon, Science 149, 754–756 (1965)
R.J. Samulski, K.I. Berns, M. Tan, N. Muzyczka, Proc. Natl. Acad. Sci. USA 79, 2077–2081 (1982)
R.J. Samulski, A. Srivastava, K.I. Berns, N. Muzyczka, Cell 33, 135–143 (1983)
D. Grimm, M.A. Kay, Curr. Gene Ther. 3, 281–304 (2003)
M. Agbandje-McKenna, J. Kleinschmidt, Methods Mol. Biol. 807, 47–92 (2011)
D. Grimm, S. Zolotukhin, Mol. Ther. 23, 1819–1831 (2015)
M.A. Kotterman, D.V. Schaffer, Nat. Rev. Genet. 15, 445–451 (2014)
M. Bartel, D. Schaffer, H. Buning, Front. Microbiol. 2, 204 (2011)
M.A. Bartel, J.R. Weinstein, D.V. Schaffer, Gene Ther. 19, 694–700 (2012)
D. Sen, J. Biomed. Sci. 21, 103 (2014)
D. Grimm, J.S. Lee, L. Wang, T. Desai, B. Akache, T.A. Storm, M.A. Kay, J. Virol. 82, 5887–5911 (2008)
W. Li, A. Asokan, Z. Wu, T. Van Dyke, N. DiPrimio, J.S. Johnson, L. Govindaswamy, M. Agbandje-McKenna, S. Leichtle, D.E. Redmond Jr., T.J. McCown, K.B. Petermann, N.E. Sharpless, R.J. Samulski, Mol. Ther. 16, 1252–1260 (2008)
J.T. Koerber, J.H. Jang, D.V. Schaffer, Mol. Ther. 16, 1703–1709 (2008)
L. Perabo, J. Endell, S. King, K. Lux, D. Goldnau, M. Hallek, H. Buning, J. Gene Med. 8, 155–162 (2006)
N. Maheshri, J.T. Koerber, B.K. Kaspar, D.V. Schaffer, Nat. Biotechnol. 24, 198–204 (2006)
L. Perabo, D. Goldnau, K. White, J. Endell, J. Boucas, S. Humme, L.M. Work, H. Janicki, M. Hallek, A.H. Baker, H. Buning, J. Virol. 80, 7265–7269 (2006)
O.J. Muller, F. Kaul, M.D. Weitzman, R. Pasqualini, W. Arap, J.A. Kleinschmidt, M. Trepel, Nat. Biotechnol. 21, 1040–1046 (2003)
E. Zinn, S. Pacouret, V. Khaychuk, H.T. Turunen, L.S. Carvalho, E. Andres-Mateos, S. Shah, R. Shelke, A.C. Maurer, E. Plovie, R. Xiao, L.H. Vandenberghe, Cell Rep. 12, 1056–1068 (2015)
J. Santiago-Ortiz, D.S. Ojala, O. Westesson, J.R. Weinstein, S.Y. Wong, A. Steinsapir, S. Kumar, I. Holmes, D.V. Schaffer, Gene Ther. 22, 934–946 (2015)
A.C. Nathwani, U.M. Reiss, E.G. Tuddenham, C. Rosales, P. Chowdary, J. McIntosh, M. Della Peruta, E. Lheriteau, N. Patel, D. Raj, A. Riddell, J. Pie, S. Rangarajan, D. Bevan, M. Recht, Y.M. Shen, K.G. Halka, E. Basner-Tschakarjan, F. Mingozzi, K.A. High, J. Allay, M.A. Kay, C.Y. Ng, J. Zhou, M. Cancio, C.L. Morton, J.T. Gray, D. Srivastava, A.W. Nienhuis, A.M. Davidoff, N. Engl. J. Med. 371, 1994–2004 (2014)
A.C. Nathwani, E.G. Tuddenham, S. Rangarajan, C. Rosales, J. McIntosh, D.C. Linch, P. Chowdary, A. Riddell, A.J. Pie, C. Harrington, J. O’Beirne, K. Smith, J. Pasi, B. Glader, P. Rustagi, C.Y. Ng, M.A. Kay, J. Zhou, Y. Spence, C.L. Morton, J. Allay, J. Coleman, S. Sleep, J.M. Cunningham, D. Srivastava, E. Basner-Tschakarjan, F. Mingozzi, K.A. High, J.T. Gray, U.M. Reiss, A.W. Nienhuis, A.M. Davidoff, N. Engl. J. Med. 365, 2357–2365 (2011)
J. Suzuki, K. Hashimoto, R. Xiao, L.H. Vandenberghe, M.C. Liberman, Sci. Rep. 7, 45524 (2017)
L.D. Landegger, B. Pan, C. Askew, S.J. Wassmer, S.D. Gluck, A. Galvin, R. Taylor, A. Forge, K.M. Stankovic, J.R. Holt, L.H. Vandenberghe, Nat. Biotechnol. 35, 280–284 (2017)
B. Pan, C. Askew, A. Galvin, S. Heman-Ackah, Y. Asai, A.A. Indzhykulian, F.M. Jodelka, M.L. Hastings, J.J. Lentz, L.H. Vandenberghe, J.R. Holt, G.S. Geleoc, Nat. Biotechnol. 35, 264–272 (2017)
K. Adachi, T. Enoki, Y. Kawano, M. Veraz, H. Nakai, Nat. Commun. 5, 3075 (2014)
M. Naumer, F. Sonntag, K. Schmidt, K. Nieto, C. Panke, N.E. Davey, R. Popa-Wagner, J.A. Kleinschmidt, J. Virol. 86, 13038–13048 (2012)
F. Sonntag, K. Schmidt, J.A. Kleinschmidt, Proc. Natl. Acad. Sci. USA. 107, 10220–10225 (2010)
L.F. Earley, J.M. Powers, K. Adachi, J.T. Baumgart, N.L. Meyer, Q. Xie, M.S. Chapman, H. Nakai, J. Virol. 91, e01980 (2017)
D. Marsic, H.R. Mendez-Gomez, S. Zolotukhin, Mol. Ther. Methods Clin. Dev. 2, 15041 (2015)
J. Korbelin, T. Sieber, S. Michelfelder, L. Lunding, E. Spies, A. Hunger, M. Alawi, K. Rapti, D. Indenbirken, O.J. Muller, R. Pasqualini, W. Arap, J.A. Kleinschmidt, M. Trepel, Mol. Ther. 24, 1050–1061 (2016)
J. Bennett, M. Ashtari, J. Wellman, K.A. Marshall, L.L. Cyckowski, D.C. Chung, S. McCague, E.A. Pierce, Y. Chen, J.L. Bennicelli, X. Zhu, G.S. Ying, J. Sun, J.F. Wright, A. Auricchio, F. Simonelli, K.S. Shindler, F. Mingozzi, K.A. High, A.M. Maguire, Sci. Transl. Med. 4, 120115 (2012)
J. Bennett, J. Wellman, K.A. Marshall, S. McCague, M. Ashtari, J. DiStefano-Pappas, O.U. Elci, D.C. Chung, J. Sun, J.F. Wright, D.R. Cross, P. Aravand, L.L. Cyckowski, J.L. Bennicelli, F. Mingozzi, A. Auricchio, E.A. Pierce, J. Ruggiero, B.P. Leroy, F. Simonelli, K.A. High, A.M. Maguire, Lancet 388, 661–672 (2016)
S.J. Chen, J. Johnston, A. Sandhu, L.T. Bish, R. Hovhannisyan, O. Jno-Charles, H.L. Sweeney, J.M. Wilson, Hum. Gene. Ther. Methods 24, 270–278 (2013)
M.K. Chuah, I. Petrus, P. De Bleser, C. Le Guiner, G. Gernoux, O. Adjali, N. Nair, J. Willems, H. Evens, M.Y. Rincon, J. Matrai, M. Di Matteo, E. Samara-Kuko, B. Yan, A. Acosta-Sanchez, A. Meliani, G. Cherel, V. Blouin, O. Christophe, P. Moullier, F. Mingozzi, T. VandenDriessche, Mol. Ther. 22, 1605–1613 (2014)
J.C. Giering, D. Grimm, T.A. Storm, M.A. Kay, Mol. Ther. 16, 1630–1636 (2008)
J. Xie, S.L. Ameres, R. Friedline, J.H. Hung, Y. Zhang, Q. Xie, L. Zhong, Q. Su, R. He, M. Li, H. Li, X. Mu, H. Zhang, J.A. Broderick, J.K. Kim, Z. Weng, T.R. Flotte, P.D. Zamore, G. Gao, Nat. Methods 9, 403–409 (2012)
A. Geisler, A. Jungmann, J. Kurreck, W. Poller, H.A. Katus, R. Vetter, H. Fechner, O.J. Muller, Gene Ther. 18, 199–209 (2011)
C. Qiao, Z. Yuan, J. Li, B. He, H. Zheng, C. Mayer, J. Li, X. Xiao, Gene Ther. 18, 403–410 (2011)
M. Davidsson, P. Diaz-Fernandez, O.D. Schwich, M. Torroba, G. Wang, T. Bjorklund, Sci. Rep. 6, 37563 (2016)
A. Barzel, N.K. Paulk, Y. Shi, Y. Huang, K. Chu, F. Zhang, P.N. Valdmanis, L.P. Spector, M.H. Porteus, K.M. Gaensler, M.A. Kay, Nature 517, 360–364 (2015)
R. Sharma, X.M. Anguela, Y. Doyon, T. Wechsler, R.C. DeKelver, S. Sproul, D.E. Paschon, J.C. Miller, R.J. Davidson, D. Shivak, S. Zhou, J. Rieders, P.D. Gregory, M.C. Holmes, E.J. Rebar, K.A. High, Blood 126, 1777–1784 (2015)
D.J. Landau, E.D. Brooks, P. Perez-Pinera, H. Amarasekara, A. Mefferd, S. Li, A. Bird, C.A. Gersbach, D.D. Koeberl, Mol. Ther. 24, 697–706 (2016)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
No research on animals or humans was performed.
Additional information
Edited by Anja Ehrhardt and Florian Kreppel.
Rights and permissions
About this article
Cite this article
Weinmann, J., Grimm, D. Next-generation AAV vectors for clinical use: an ever-accelerating race. Virus Genes 53, 707–713 (2017). https://doi.org/10.1007/s11262-017-1502-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11262-017-1502-7