Abstract
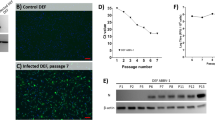
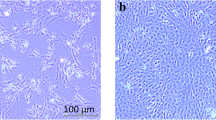
Boid inclusion body disease (BIBD) is a viral disease of boids caused by reptarenavirus. In this study, tissue from naturally infected boid snakes were homogenized and propagated in African Monkey kidney (Vero) and rat embryonic fibroblast (REF) cells. Virus replication was determined by the presence of cytopathic effect, while viral morphology was observed using transmission electron microscopy. Viral RNA was amplified using RT-PCR with primers specific for the L-segment of reptarenavirus; similarly, quantification of viral replication was done using qPCR at 24–144 h postinfection. Viral cytopathology was characterized by cell rounding and detachment in both Vero and REF cells. The viral morphology showed round-to-pleomorphic particles ranging from 105 to 150 nm which had sand-like granules. Sanger sequencing identified four closely associated reptarenavirus species from 15 (37.5 %) of the total samples tested, and these were named as follows: reptarenavirus UPM-MY 01, 02, 03, and 04. These isolates were phylogenetically closely related to the University Helsinki virus (UHV), Boa Arenavirus NL (ROUTV; BAV), and unidentified reptarenavirus L20 (URAV-L20). Comparison of deduced amino acid sequences further confirmed identities to L-protein of UHV, L-polymerase of BAV and RNA-dependent RNA polymerase of URAV-L20. Viral replication in Vero cells increased steadily from 24 to 72 h and peaked at 144 h. This is the first study in South East Asia to isolate and characterize reptarenavirus in boid snakes with BIBD.






Similar content being viewed by others
References
T. Aqrawi, A. Stöhr, T. Knauf-Witzens, A. Krengel, K. Heckers, R. Marschang, Identification of snake arenaviruses in live boas and pythons in a zoo in Germany. Tierärztliche Praxis Kleintiere 43(4), 239–247 (2015)
R. Bodewes, M. Kik, V.S. Raj, C. Schapendonk, B. Haagmans, S.L. Smits, A. Osterhaus, Detection of novel divergent arenaviruses in boid snakes with inclusion body disease in The Netherlands. J. Gen. Virol. 94(6), 1206–1210 (2013)
R. Bodewes, V.S. Raj, M.J. Kik, C.M. Schapendonk, B.L. Haagmans, S.L. Smits, A.D. Osterhaus, Updated phylogenetic analysis of arenaviruses detected in boid snakes. J. Virol. 88(2), 1399–1400 (2014)
L.-W. Chang, E.R. Jacobson, Inclusion body disease, a worldwide infectious disease of boid snakes: a review. J. Exot. Pet Med. 19(3), 216–225 (2010)
H. Fraenkel-Conrat, R.R. Wagner, Viral Cytopathology: Cellular Macromolecular Synthesis and Cytocidal Viruses Including a Cumulative Index to the Authors and Major Topics Covered in Volumes 1–19, vol. 19 (Springer Science & Business Media, Berlin, 2012)
J. Hepojoki, P. Salmenperä, T. Sironen, U. Hetzel, Y. Korzyukov, A. Kipar, O. Vapalahti O, Arenavirus coinfections are common in snakes with boid inclusion body disease. J. Virol. 89(16), 8657–8660 (2015)
U. Hetzel, T. Sironen, P. Laurinmäki, L. Liljeroos, A. Patjas, H. Henttonen, A. Vaheri, A. Artelt et al., Isolation, identification, and characterization of novel arenaviruses, the etiological agents of boid inclusion body disease. J. Virol. 87(20), 10918–10935 (2013)
U. Hetzel, T. Sironen, P. Laurinmäki, L. Liljeroos, A. Patjas, H. Henttonen, A. Vaheri, A. Artelt et al., Reply to “Updated Phylogenetic Analysis of Arenaviruses Detected in Boid Snakes”. J. Virol. 88(2), 1401 (2014)
A.N. Honko, P.B. Jahrling, J.H. Kuhn, S.R. Radoshitzky, J.C. Johnson, Arenaviruses Global Virology I-Identifying and Investigating Viral Diseases (Springer, Berlin, 2015), pp. 501–541
E. Jacobson, Infectious Diseases and Pathology of Reptiles: Color Atlas and Text (CRC Press, Boca Raton, 2010)
E.R. Jacobson, J. Orós, S.J. Tucker, D.P. Pollock, K.L. Kelley, R.J. Munn, B.A. Lock, A. Mergia et al., Partial characterization of retroviruses from boid snakes with inclusion body disease. Am. J. Vet. Res. 62(2), 217–224 (2001)
H.M. Lander, A.M. Grant, T. Albrecht, T. Hill, C.J. Peters, Endothelial cell permeability and adherens junction disruption induced by Junín Virus Infection. Am. J. Trop. Med. Hyg. 90(6), 993–1002 (2014)
L. McLay, Y. Liang, H. Ly, Comparative analysis of disease pathogenesis and molecular mechanisms of New World and Old World arenavirus infections. J. Gen. Virol. 95(Pt 1), 1–15 (2014)
S.R. Radoshitzky, Y. Bào, M.J. Buchmeier, R.N. Charrel, A.N. Clawson, C.S. Clegg, J.L. DeRisi, S. Emonet et al., Past, present, and future of arenavirus taxonomy. Arch. Virol. 160(7), 1851–1874 (2015)
M. Salvato, The Arenaviridae (Springer Science & Business Media, Berlin, 2012)
M. Stenglein, E. Jacobson, J. DeRisi, Recombination, reassortment, and many-to-one genotypes in natural arenavirus infections. New Horiz. Transl. Med. 2(4), 133–134 (2015)
M.D. Stenglein, C. Sanders, A.L. Kistler, J.G. Ruby, J.Y. Franco, D.R. Reavill, F. Dunker, J.L. DeRisi, Identification, characterization, and in vitro culture of highly divergent arenaviruses from boa constrictors and annulated tree boas: candidate etiological agents for snake inclusion body disease. MBio. 3(4), e00180–12 (2012). doi:10.1128/mBio.00180-12
A.P. Turchetti, H.P. Tinoco, M. Malta, M. da Costa, A.T. Pessanha, S.A. Soave, T.A. Paixão, R.L. Santos, Inclusion body disease in a Corallus hortulanus. Braz. J. Vet. Pathol. 6(1), 15–18 (2013)
J.C. Zapata, M.S. Salvato, Arenavirus variations due to host-specific adaptation. Viruses 5(1), 241–278 (2013)
Acknowledgments
The authors are grateful for the funding received under the Universiti Putra Malaysia Postgraduate Grant Scheme (GP/IPS/2014/9438740) and the Ministry of Higher Education Malaysia, Fundamental Research Grant Scheme (FRGS/2014/STWN03/UPM/01/2).
Author contribution
All authors contributed equally to this work and have read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest regarding this work.
Ethical approval
This article does not contain any studies performed on live animals by any of the authors. Snakes used for the study were carcasses brought in dead (BID) to the necropsy unit.
Additional information
Edited by Zhen F. Fu.
Rights and permissions
About this article
Cite this article
Abba, Y., Hassim, H., Hamzah, H. et al. In vitro isolation and molecular identification of reptarenavirus in Malaysia. Virus Genes 52, 640–650 (2016). https://doi.org/10.1007/s11262-016-1345-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11262-016-1345-7