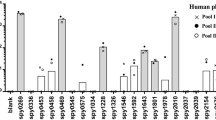
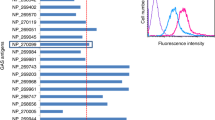
Abstract
Streptococcus canis (S. canis), a lancefield group G streptococcus, is an opportunistic pathogen mainly found in dogs and cats. The study on pathogenesis and protective immune mechanism of S. canis is not clear. A new streptococcal protective antigen (SPA) was first identified from a genomic library of S. canis. SPA of S. canis (SPASc) contained a 1224-bp open reading frame which encoded a 407aa protein and a 34-aa signal sequence with a deduced molecular mass of 46.368 kDa. Protein analysis and BLAST result showed that SPASc was homologous to the SPA of Streptococcus. equi subsp. zooepidemicus, M protein Streptococcus. equi., and SPA of Streptococcus pyogenes. The protective response of SPASc antiserum was demonstrated by passive mouse protection. These studies suggested that SPASc might be an important component of vaccines to prevent S. canis infections.



Similar content being viewed by others
References
Broeseker TA, Boyle MDP, Lottenberg R (1988) Characterization of the interaction of human plasmin with its specific receptor on a group A streptococcus. Microb. Pathog. 5, 19–27.
Cunningham WM (2000) Pathogenesis of Group A Streptococcal Infections. Clinical Microbiology Reviews 13, 470–511.
Dale JB, Chiang EY, Liu S, et al (1999) New protective antigen of group A streptococci. Journal of Clinical Investigation. 103, 1231–1268.
Devriese LA, Hommez J, Kilpper-Balz R, Schleifer KH (1986) Streptococcus canis sp. nov.: a species of group G streptococci from animals. Int. J. Syst. Bacteriol. 36, 422–425.
DeWinter LM, Low DE, Prescott J F (1999) Virulence of Streptococcus canis from canine streptococcal toxic shock syndrome and necrotizing fasciitis. Vet. Microbiol. 70, 95–110.
Galpérine T, Cazorla C, Elodie B, et al (2007)Streptococcus canis infections in humans: Retrospective study of 54 patients. Journal of Infection 55, 23–26.
Hassan AA, Akineden O, Usleber E (2005)Identification of Streptococcus canis isolated from milk of dairy cows with subclinical mastitis. J Clin Microbiol. 43, 1234–1238.
Ji Y, McLandsborough L, Kondagunta A, Cleary PP (1996) C5a peptidase alters clearance and trafficking of group A streptococci by infected mice. Infect. Immun. 64, 503–510.
Lam MM, Clarridge JE 3rd, Young EJ (2007)The other group G Streptococcus: increased detection of Streptococcus canis ulcer infections in dog owners. J Clin Microbiol. 45, 2327–2329.
Lancefield RC (1962) Current knowledge of the type-specific M antigens of group A streptococci. J. Immunol. 89, 307–313.
Lottenberg R, Minning-Wenz D, Boyle M (1994) Capturing host plasmin(ogen): a common mechanism for invasive pathogens. Trends Microbiol. 2, 20–24.
Miller CW, Prescot JF, Mathews KA, et al (1996) Streptococcal toxic shock syndrome in dogs. J. Am. Vet. Med. Assoc. 209, 1421–1426.
Musser JM, Stockbauer K, Kapur V, et al (1996)Substitution of cysteine 192 in a highly conserved Streptococcus pyogenes extracellular cysteine protease (interleukin 1b convertase) alters proteolytic activity and ablates zymogen processing. Infect. Immun. 64, 1913–1917.
Parkhill J, Sebaihia M, Preston A, et al (2003) Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat. Genet. 35, 32–40.
Tikofsky LL, Zadoks RN (2005) Cross-infection between cats and cows: origin and control of Streptococcus canis mastitis in a dairy herd. J Dairy Sci. 88, 2707–2713.
Timoney JF, Artiushin S, Boschwitz JS (1997) Comparison of the Sequences and Functions of Streptococcus equi M-Like Proteins SeM and SzPSe. Infect. Immun. 65, 3600–3605.
Timoney JF, Yang J, Liu J, et al (2008) IdeE reduces the bactericidal activity of equine neutrophils for Streptococcus equi. Veterinary Immunology and Immunopathology 122, 76–82.
Verma A, Artiushin S, Timoney JF (2005) LruA and LruB, novel lipoproteins of pathogenic leptospira interrogans associated with equine recurrent uveitis. Infect Immun. 73, 7259–7266.
Wessels MR, Bronze MS (1994) Critical role of the group A streptococcal capsule in pharyngeal colonization and infection in mice. Proc. Natl. Acad. Sci. USA. 91, 12238–12242.
Whatmore AM, Engler KH, Gudmundsdottir G, et al (2001) Identification of Isolates of Streptococcus canis Infecting Humans. J Clin Microbiol. 39, 4196–4199.
Acknowledgments
The financial supports of Tianjin Natural Science Foundation (07JCYBJC16000) and the Project-sponsored by SRF for ROCS, SEM were gratefully acknowledged. We thank Dr. Wenzhi Xue of Intervet (Merrian, Kansas) and Dr. Minghao Sun of the Scripps Research Institute for proof reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. 1
DNA sequnence and deduced amino acid of SPASc. The sequence contains complete open reading frame with 1,224 nucleotides and deduced 407 amino acids. Underlined sequence means signal sequence. The sequence has been submitted to GeneBank and has the accession no. ACM47242 (DOC 69 kb)
Rights and permissions
About this article
Cite this article
Yang, J., Liu, Y., Xu, J. et al. Characterization of a new protective antigen of Streptococcus canis . Vet Res Commun 34, 413–421 (2010). https://doi.org/10.1007/s11259-010-9414-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-010-9414-1