Abstract
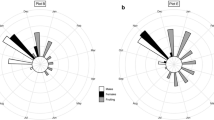
As deforestation and land-use/land-cover change advance in tropical forest regions, an understanding of how plants adjust phenology and reproductive dynamics to altered landscapes can provide insights into plasticity, productivity, and population persistence. We compared the reproductive phenology, sex expression, and flower and fruit production of two monoecious Amazonian palms, Attalea phalerata and Attalea speciosa, in old-growth forest and as remnant trees growing in actively grazed pastures. Using 2 years of phenology data collected from natural populations near Vila Extrema, Rondônia, and eastern Acre, Brazil, we compared flowering and fruiting in the two habitats and tested for effects of palm height, crown size, and light availability on inflorescence and sex expression. Forest conversion to pasture stimulated greater overall flowering and fruiting in individual Attalea palms. As a population, remnant pasture palms continuously bore flowers and fruits year-round, while forest palms flowered seasonally in isolated peaks with consecutive months of inactivity. Crown size and greater light exposure affected flowering and fruiting dynamics in A. phalerata and A. speciosa, respectively, and increased light availability shifted A. speciosa sex expression towards greater female investment, primarily through regulation of sex determination and bud abortion. Removal of tropical forest does not always lead to the downfall of remnant tree populations, and under favorable conditions, such as abandonment of cropland and pasture, higher levels of reproduction can facilitate recovery of future generations. Tree species with flexible sex expression may be particularly resilient in the face of land-use and land-cover change.





Similar content being viewed by others
References
Abramovitz JN, Mattoon AT (1999) Reorienting the forest products economy. In: Starke L (ed) State of the world. WW Norton and Co., New York, pp 60–77, 206–213
Adam H, Collin M, Richaud F, Beulé T, Cros D, Omeré A, Nodichao L, Nouy B, Tregear JW (2011) Environmental regulation of sex determination in oil palm: current knowledge and insights from other species. Ann Bot 108:1529–1537
Adler GH, Lambert TD (2008) Spatial and temporal variation in the fruiting phenology of palms in isolated stands. Plant Spec Biol 23:9–17
Agostinelli C, Lund U (2017) R package ‘circular’: circular statistics (version 0.4-93) https://r-forge.r-project.org/projects/circular/
Aldrich PR, Hamrick JL (1998) Reproductive dominance of pasture trees in a fragmented tropical forest mosaic. Science 281:103–105
Anderson AB (1983) The biology of Orbignya martiana (Palmae), a tropical dry forest dominant in Brazil. Dissertation, University of Florida
Anderson AB, Overal WL, Henderson A (1988) Pollination ecology of a forest-dominant palm (Orbignya phalerata Mart.) in Northern Brazil. Biotropica 20:192–205
Anderson AB, May PH, Balick MJ (1991) The subsidy from nature: palm forests, peasantry, and development on an Amazon frontier. Columbia University Press, New York
Andreazzi CS, Pires AS, Pimenta CS, Fernandez FAS (2012) Increased female reproduction favours the large-seeded palm Attalea humilis in small Atlantic Forest fragments. J Trop Ecol 28:321–325
Anthelme F, Lincango J, Gully C, Duarte N, Montufar R (2011) How anthropogenic disturbances affect the resilience of a keystone palm tree in the threatened Andean cloud forest? Biol Conserv 144:1059–1067
Athayde EA, Morellato LPC (2014) Anthropogenic edges, isolation and the flowering time and fruit set of Anadenanthera peregrina, a cerrado savanna tree. Int J Biometeorol 58:443–454
Baker WJ, Hutton I (2006) Lepidorrhachis. Palms 50:33–38
Barot S, Mitja D, Miranda I, Meija GD, Grimaldi M (2005) Reproductive plasticity in an Amazonian palm. Evol Ecol Res 7:1051–1065
Batschelet E (1981) Circular statistics for biology. Academic Press, London
Bazzaz FA, Carlson RW (1982) Photosynthetic acclimation to variability in the light environment of early and late successional plants. Oecologia 54:313–316
Bechtold WA (2003) Crown position and light exposure classification—an alternative to field-assigned crown class. N J Appl For 20:154–160
Berry EJ, Gorchov DL (2006) Female fecundity is dependent on substrate, rather than male abundance, in the wind-pollinated, dioecious understory palm Chamaedorea radicalis. Biotropica 39:186–194
Bierzychudek P (1984) Determinants of gender in Jack-in-the-Pulpit: the influence of plant size and reproductive history. Oecologia 65:14–18
Borchert R, Meyer SA, Felger RS, Porter-Bolland L (2004) Environmental control of flowering periodicity in Costa Rican and Mexican tropical dry forests. Global Ecol Biogeogr 13:409–425
Breed MF, Ottewell KM, Gardner MG, Lowe AJ (2011) Clarifying climate change adaptation responses for scattered trees in modified landscapes. J Appl Ecol 48:637–641
Buide ML, del Valle JC, Castilla AR, Narbona E (2018) Sex expression variation in response to shade in gynodioecious-gynomonoecious species: Silene littorea decreases flower production and increases female flower proportion. Environ Exp Bot 146:54–61
Burd M (1994) Bateman’s principle and plant reproduction: the role of pollen limitation in fruit and seed set. Bot Rev 60:83–139
Burgess VJ, Kelly D, Robertson A, Ladley JJ (2006) Positive effects of forest edges on plant reproduction: literature review and a case study of bee visitation to flowers of Peraxilla tetrapetala (Loranthaceae). New Zeal J Ecol 30:179–190
Cardoso FCG, Zwiener VP, Marques MCM (2018) Tree phenology along a successional gradient of tropical Atlantic Forest. J Plant Ecol. https://doi.org/10.1093/jpe/rty020
Cascante A, Quesada M, Lobo JJ, Fuchs EA (2002) Effects of dry tropical forest fragmentation on the reproductive success and genetic structure of the tree Samanea saman. Conserv Biol 16:137–147
Castro ER, Galetti M, Morellato LPC (2007) Reproductive phenology of Euterpe edulis (Arecaceae) along a gradient in the Atlantic rainforest of Brazil. Aust J Bot 55:725–735
Charnov EL (1982) The theory of sex allocation. Princeton University Press, Princeton
Charnov EL, Bull J (1977) When is sex environmentally determined? Nature 266:828–830
Chazdon R (1986) Light variation and carbon gain in rain forest understory palms. J Ecol 74:995–1012
Chazdon R, Pearcy R, Lee D, Fetcher N (1996) Photosynthetic responses of tropical plants to contrasting light environments. In: Mulkey SS, Chazdon RL, Smith AP (eds) Tropical forest plant ecophysiology. Chapman & Hall, New York, NY, pp 5–55
Clay K (1993) Size dependent gender change in green dragon (Arisaema dracontium; Araceae). Am J Bot 80:769–777
Cobb NS, Trotter RT III, Whitham TB (2002) Long-term sexual allocation in herbivore resistant and susceptible pinyon pine (P. edulis). Oecologia 130:78–87
Cortés-Flores J, Hernández-Esquivel KB, González-Rodríguez A, Ibarra-Manríquez G (2017) Flowering phenology, growth forms, and pollination syndromes in tropical dry forest species: influence of phylogeny and abiotic factors. Am J Bot 104:39–49
Cruden RW (1988) Temporal dioecism: systematic breadth, associated traits, and temporal patterns. Bot Gaz 149:1–15
Cruden RW, Hermann-Parker SM (1977) Temporal dioecism: an alternative to dioecism. Evolution 31:863–866
Cunningham SA (1997) The effect of light environment, leaf area, and stored carbohydrates on inflorescence production by a rain forest understory palm. Oecologia 11:36–44
Desteven D, Windsor DM, Putz FE, De Leon B (1987) Vegetative and reproductive phenologies of a palm assemblage in Panama. Biotropica 19:342–356
Dick CW (2001) Genetic rescue of remnant tropical trees by an alien pollinator. Proc Biol Sci 268:2391–2396
Fava WS, Covre W, Sigrist MR (2011) Attalea phalerata and Bactris glaucescens (Arecaceae, Arecoideae): phenology and pollination ecology in Panatanal, Brazil. Flora 206:575–584
Feil JP (1996) Fruit production of Attalea colenda (Arecaceae) in coastal Ecuador—an alternative oil resource? Econ Bot 5:300–309
Fischer J, Stott J, Law BS (2010) The disproportionate value of scattered trees. Biol Conserv 143:1564–1567
Fox JF (1993) Size and sex allocation in monoecious woody plants. Oecologia 94:110–113
Fox J, Weisberg S (2011) An R companion to applied regression, 2nd edn. Sage, Thousand Oaks, CA
Freeman DC, Harper KT, Charnov EL (1980) Sex change in plants: old and new observations and new hypotheses. Oecologia 47:222–232
Freeman DC, McArthur ED, Harper KT, Blauer AC (1981) Influence of environment on the floral sex ratio on monoecious plants. Evolution 35:194–197
Friedman J, Barrett SCH (2009) The consequences of monoecy and protogyny for mating in wind-pollinated Carex. New Phytol 181:489–497
Fuchs EJ, Lobo JA, Quesada M (2003) Effects of forest fragmentation and flowering phenology on the reproductive success and mating patterns of the tropical dry forest tree Pachira quinata. Conserv Biol 17:149–157
Grogan J, Schulze M, Galvão J (2010) Survival, growth and reproduction by big-leaf mahogany (Swietenia macrophylla) in open clearing vs. forested conditions in Brazil. New Forest 40:335–347
Grupo de Estudos e Serviços Ambientais. http://www.acrebioclima.pro.br. Accessed 20 Mar 2018
Haddad NM, Brudvig LA, Clobert J, Davies KF, Gonzalez A, Holt RD, Lovejoy TE, Sexton JO, Austin MP, Collins CD, Cook WM, Damschen EI, Ewers RM, Foster BL, Jenkins CN, King AJ, Laurance WF, Levey DJ, Margules CR, Melbourne BA, Nicholls AO, Orrock JL, Song DX, Townshend JR (2015) Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci Adv 1:e1500052
Hartley CWS (1977) The oil palm. Longman, London
Harvey CA, Haber WA (1999) Remnant trees and the conservation of biodiversity in Costa Rican pastures. Agrofor Syst 44:37–68
Henderson A (1995) The palms of the Amazon. Oxford University Press, New York
Henderson A (2002) Evolution and ecology of palms. The New York Botanical Garden Press, Brooklyn
Henderson A, Galeano G, Bernal R (1995) Field guide to the palms of the Americas. Princeton University Press, Princeton
Henson IE (2000) Modelling the effects of ‘haze’ on oil palm productivity and yield. J Oil Palm Res 12:123–134
Herrerias-Diego Y, Quesada M, Stoner KE, Lobo JA (2006) Effects of forest fragmentation on phenological patterns and reproductive success of the tropical dry forest tree Ceiba aesculifolia. Conserv Biol 20:1111–1120
Holdridge LR (1978) Life zone ecology. Centro Cientifico Tropical, San José
INMET (Instituto Nacional de Meterologia) (2008) Ministerio da Agricultura, Pecuaria e Abastecimento, Brasilia, Brazil. http://www.inmet.gov.br Accessed 20 Nov 2009
Jackman S (2017) Pscl: classes and methods for R developed in the political science computations laboratory. United States Studies Centre, University of Sydney, Sydney
Janzen DH (1986) The future of tropical ecology. Annu Rev Ecol Syst 17:305–324
Jones LH (1997) The effects of leaf pruning and other stresses on sex determination in the Oil Palm and their representation by a computer simulation. J Theor Biol 187:241–260
Klinkhamer PGL, De Jong RJ, Metz H (1997) Sex and size in cosexual plants. Trends Ecol Evol 12:260–265
Korpelainen H (1998) Labile sex expression in plants. Biol Rev 73:157–180
Kuchmeister H, Gottsberger F, Silberbauer-Gottsberger I (1993) Pollination biology of Orbignya spectabilis, a “monoecious” Amazonian palm. In: Barthlott W, Naumann C, Schmidt-Loske C, Schuchmann K (eds) Animal-plant interactions in tropical environments. Zoologisches Forschungsinstitut und Museum Alexander Koenig, Bonn, pp 67–76
Lander TA, Boshier DH, Harris SA (2010) Fragmented but not isolated: contribution of single trees, small patches and long-distance pollen flow to genetic connectivity for Gomortega keule, an endangered Chilean tree. Biol Conser 143:2583–2590
Lara CE, Díez MC, Restrepo Z, Núñez LA, Moreno F (2017) Flowering phenology and flower visitors of the Macana Palm Wettinia kalbreyeri (Arecaceae) in an Andean montane forest. Rev Mex Biodivers 88:106–112
Laurance WF, Rankin de Merona JM, Andrade A, Laurance SG, D’Angelo S, Lovejoy TE, Vasconcelos HL (2003) Rain-forest fragmentation and the phenology of Amazonia tree communities. J Trop Ecol 19:343–347
Lazaro A, Mendez M (2007) Variation in sexual expression in a monoecious shrub Buxus balearica at different scales. Plant Biol 9:736–744
Legros S, Mialet-Serra I, Calioman J-P, Siregar FA, Clement-Vidal A, Dingkuhn M (2009) Phenology and growth adjustments for oil palm (Elaeis guineensis) to photoperiod and climate variability. Ann Bot 104:1171–1182
Levin DA (2009) Flowering-time plasticity facilitates niche shifts in adjacent populations. New Phytol 183:661–666
Lloyd DG (1979) Parental strategies of angiosperms. New Zeal J Bot 17:595–606
Lloyd DG, Bawa KS (1984) Modification of the gender of seed plants in varying conditions. Evol Biol 17:255–339
Mann LK (1942) Effects of photoperiod on sex expression in Ambrosia trifida. Bot Gaz 103:780–787
Manning AD, Fischer J, Lindenmayer DB (2006) Scattered trees are keystone structures—implications for conservation. Biol Conserv 132:311–321
Marcus J (2010) Observations on the flowering of Marojejya darianii. Palms 54:189–192
McKechnie IM, Sargent RD (2013) Do plant traits influence a species’ response to habitat disturbance? A meta-analysis. Biol Conserv 168:69–77
Millerón M, López de Heredia U, Lorenzo Z, Perea R, Dounavi A, Alonso J, Gil L, Nanos N (2012) Effect of canopy closure on pollen dispersal in a wind-pollinated species (Fagus sylvatica L.). Plant Ecol 213:1715–1728
Mitja D, Ferraz IDK (2001) Establishment of babassu in pastures in Para, Brazil. Palms 45:138–147
Montufar R, Anthelme F, Pintaud J-C, Balslev H (2011) Distrubance and resilience in tropical American palm populations and communities. Bot Rev 77:426–461
Morellato LPC, Talora DC, Takahasi A, Bencke CC, Romera EC, Zipparro VB (2000) Phenology of Atlantic rain forest trees: a comparative study. Biotropica 32:811–823
Morellato LPC, Alberti LF, Hudson IL (2010) Applications of circular statistics in plant phenology: a case studies approach. In: Hudson IL, Keatley MR (eds) Phenological research: methods for environmental and climate change analysis. Springer, Netherlands, pp 339–359
Nadot S, Alapetite E, Baker WJ, Tregear JW, Barfod AS (2016) The palm family (Arecaceae): a microcosm of sexual system evolution. Bot J Linn Soc 182:376–388
Nepstad DC, Uhl C, Serrão EAS (1991) Recuperation of a degraded Amazonian landscape: forest recovery and agricultural restoration. Ambio 20:248–255
Newstrom LE, Frankie GW, Baker G (1994) A new classification for plant phenology based on flowering patterns in lowland tropical rain forest trees at La Selva, Costa Rica. Biotropica 26:141–159
Olivares I, Galeano G (2013) Leaf and inflorescence production of the winde palm (Attalea butyracea) in the Dry Magdalena River Valley, Colombia. Caldasia 35:37–48
Opedal OH, Listermann J, Albertsen E, Armbruster WS, Pélabon C (2016) Multiple effects of drought on pollination and mating-system traits in Dalechampia scandens. Int J Plant Sci 177:682–693
Otero-Arnaiz A, Oyama K (2001) Reproductive phenology, seed-set and pollination in Chameadorea alternans, an understorey dioecious palm in a rain forest in Mexico. J Trop Ecol 17:745–754
Piñero D, Sarukhán J (1982) Reproductive behaviour and its individual variability in a tropical palm, Astrocaryum mexicanum. J Ecol 70:461–472
Poorter L (1999) Growth responses of 15 rain-forest tree species to a light gradient: the relative importance of morphological and physiological traits. Funct Ecol 13:396–410
Pulido MR, Caballero J (2006) The impact of shifting agriculture on the availability of non-timber forest products: the example of Sabal yapa in the Maya lowlands of Mexico. For Ecol Manag 222:399–409
Queenborough SA, Burslem DFP, Garwood NC, Valencia R (2007) Determinants of biased sex ratios and inter-sex costs of reproduction in dioecious tropical forest trees. Am J Bot 94:67–78
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rathcke B, Lacey EP (1985) Phenological patterns of terrestrial plants. Annu Rev Ecol Syst 16:179–214
Rios LD, Fuchs EJ, Hodel DR, Cascante-Marín A (2014) Neither insects nor wind: ambophily in dioecious Chamaedorea palms (Arecaceae). Plant Biol 16:702–710
Robinson D, Warmsley A, Nowakowski AJ, Reider KE, Donnelly MA (2013) The value of remnant trees in pastures for a neotropical poison frog. J Trop Ecol 29:345–352
Rocha OJ, Aguilar G (2001) Reproductive biology of the dry forest tree Enterolobium cyclocarpum (guanacaste) in Costa Rica: a comparison between trees left in pastures and trees in continuous forest. Am J Bot 88:1607–1614
Rojas-Robles R, Stiles FG (2009) Analysis of a supra-annual cycle: reproductive phenology of the palms Oenocarpus bataua in a forest of the Colombian Andes. J Trop Ecol 25:41–51
Rymer PD, Sandiford M, Harris SA, Billingham MR, Boshier DH (2013) Remnant Pachira quinata pasture trees have greater opportunities to self and suffer reduced reproductive success due to inbreeding depression. Heredity. https://doi.org/10.1038/hdy.2013.73
Sandor ME, Chazdon RL (2014) Remnant trees affect species composition but not structure of tropical second-growth forest. PLoS ONE 9:e83284
Sanin MJ, Anthelme F, Pintaud J-C, Galeano G, Bernal R (2013) Juvenile resilience and adult longevity explain residual populations of the Andean Wax Palm Ceroxylon quindiuense after deforestation. PLoS ONE 8:e74139
Scariot AO, Lleras E, Hay JD (1995) Flowering and fruiting phenologies of the palm Acrocomia aculeata: patterns and consequences. Biotropica 27:168–173
Schlawin JR, Zahawi RA (2008) ‘Nucleating’ succession in recovering neotropical wet forests: the legacy of remnant trees. J Veg Sci 19:485–492
Schroth G, da Mota MSS, Lopes R, de Freitas AF (2004) Extractive use, management and in situ domestication of a weedy palm, Astrocaryum tucuma, in the central Amazon. For Ecol Manag 202:161–179
Solomon BP (1985) Environmentally influenced changes in sex expression in an andromonoecious plant. Ecology 66:1321–1332
Stevenson PR, Castellanos MC, Cortes AI, Link A (2008) Flowering patterns in a seasonal tropical lowland forest in Western Amazonia. Biotropica 40:559–567
Thomas RG (1956) Effects of temperature and length of day on sex expression of monoecious and dioecious angiosperms. Nature 4532:552–553
Tomlinson PB (1990) The structural biology of palms. Oxford University Press, New York
Tucker Lima JM (2010) Ecology of native oil-producing palms and their potential for biofuel production in southwestern Amazonia. Dissertation, University of Florida
Urrego LE, Galeano A, Peñuela C, Sánchez M, Toro E (2016) Climate-related phenology of Mauritia flexuosa in the Colombian Amazon. Plant Ecol 217:1207–1218
Varga S, Kytöviita M-M (2016) Light availability affects sex lability in a gynodioecious plant. Am J Bot 103:1928–1936
Vega-Frutis R, Macías-Ordóñez R, Guevara R, Fromhage L (2014) Sex change in plants and animals: a unified perspective. J Evol Biol 27:667–675
Voeks RA (1987) A biogeography of the piassava fiber palm (Attalea funifera Mart.) of Bahia, Brazil. Dissertation, University of California, Berkeley
Voeks RA (1988) Changing sexual expression of a Brazilian rain forest palm (Attalea funifera Mart.). Biotropica 20:107–113
Wallraff HG (1979) Goal-oriented and compass-oriented movements of displaced homing pigeons after confinement in differentially shielded aviaries. Behav Ecol Sociobiol 5:201–225
Werren JH, Beukeboom LW (1998) Sex determination, sex ratios, and genetic conflict. Annu Rev Ecol Syst 29:233–261
White GM, Boshier DH (2000) Fragmentation in Central American dry forests—genetic impacts on Swientenia humilis. In: Young AG, Clarke GM (eds) Genetics, demography and the viability of fragmented populations. Cambridge University Press, Cambridge, UK, pp 293–311
White GM, Boshier DH, Powell W (2002) Increased pollen flow counteracts fragmentation in a tropical dry forest: an example from Swietenia humilis Zuccarini. PNAS 99:2038–2042
Wick B, Tiessen H, Menezes RSC (2000) Land quality changes following the conversion of the natural vegetation into silvo-pastoral systems in semi-arid NE Brazil. Plant Soil 222:59–70
Williams M (2008) A new look at global forest histories of land clearing. Annu Rev Env Resour 33:345–367
Williams-Linera G, Alvarez-Aquino C (2016) Vegetative and reproductive tree phenology of ecological groups in a tropical dry forest in central Veracruz, Mexico. Bot Sci 94:745–756
Xiao Y, Li X, Cao Y, Dong M (2016) The diverse effects of habitat fragmentation on plant-pollinator interactions. Plant Ecol 217:857–868
Yamasaki S, Fujii N, Takahashi H (2005) Hormonal regulation of sex expression in plants. Vitam Horm 72:79–110
Zeileis A, Hothorn R (2002) Diagnostic checking in regression relationships. R News 2:7–10
Zhang Z-Q, Zhu X-F, Sun H, Yang Y-P, Barrett SCH (2014) Size-dependent gender modification in Lilium apertum (Liliaceae): does this species exhibit genter diphasy? Ann Bot 114:441–453
Zoneamento Ecologico-Economico do Acre (2002) Recursos naturais e meio ambiente, vol. I. Secretaria de Estado de Ciencia, Tecnologia e Meio Ambiente, Rio Branco, Brazil
Acknowledgements
The authors thank Dr. Evandro Linhares Ferreira from the Instituto Nacional de Pesquisas da Amazonia (INPA) for his constant logistical and intellectual support. We are grateful to the research team at Parque Zoobotânico, Universidade Federal do Acre (UFAC), Brazil, for assistance with fieldwork, especially Evandro Lima, Plínio Mitoso, José Lira, Janice Nascimento, and Anelena Carvalho. We also thank Drs. Jack Putz, Emilio Bruna, and Jane Southworth from the University of Florida (UF) for valuable comments on earlier drafts. Research was conducted under a cooperative agreement between UF and UFAC and funded in part by the United States Environmental Protection Agency (EPA) under the Science to Achieve Results (STAR) Graduate Fellowship Program. EPA has not officially endorsed this publication and the views expressed herein may not reflect the views of the EPA. Partial financial support was also provided by an Integrative Graduate Education and Research Traineeship Program (IGERT) Fellowship from the National Science Foundation (NSF), and grants from the International Palm Society and UF Tropical Conservation and Development Program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by E.T.F. Witkowski.
Rights and permissions
About this article
Cite this article
Tucker Lima, J.M., Caruso, N.M., Clugston, J. et al. Landscape change alters reproductive phenology and sex expression in Attalea palms (Arecaceae) of southwestern Amazonia. Plant Ecol 219, 1225–1245 (2018). https://doi.org/10.1007/s11258-018-0874-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-018-0874-7