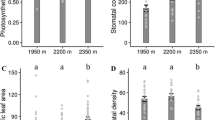
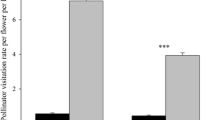
Abstract
Based on the hypothesis that both plant size and local conspecific density influence allocation to female/male functions, we explored the relationship between plant height, local conspecific density, sexual expression, and fruit production in the andromonoecious shrub Caesalpinia gilliesii. We quantified the total number of perfect and staminate flowers, the pollen received and fruits produced per plant in two populations, and estimated phenotypic gender and fruit set. Local density failed to explain phenotypic gender, nevertheless, plant height and fruit set increased with local density in one population where, in addition, the slopes for the size-dependent sex allocation curve were steeper. As observed for other plant species, this suggests that between population differences in resource availability is the main underlying factor for the observed population differences in the size-dependent allocation pattern to flowers and fruits. On the other hand, the number of staminate and perfect flowers per plant increased with plant height and the fastest increase of staminate flowers resulted in a male-biased size-dependent sex allocation strategy in both populations. Since pollination intensity was not correlated with plant height in any population, the observed allocation strategy cannot be attributed to differences in pollen availability between different sized individuals, but to differences in plant size. Finally, because fruit set and total fruit number increased with plant height in one population, the obtained results provide further evidence that animal-pollinated, andromonoecious species may exhibit a male-biased size-dependent sex allocation strategy, which may favor female fecundity.



Similar content being viewed by others
References
Bertin RI (1982) The evolution and maintenance of andromonoecy. Evol Theory 6:25–32
Bickel AM, Freeman DC (1993) Effects of pollen vector and plant geometry on floral sex ratio in monoecious plants. Am Midl Nat 130:239–247
Bosch M, Waser NM (2001) Experimental manipulation of plant density and its effect on pollination and reproduction of two confamilial montane herbs. Oecologia 126:76–83. doi:10.1007/s004420000488
Burd M, Callahan HS (2000) What does the male function hypothesis claim? J Evol Biol 13:735–742
Cabrera AL (1994) Regiones fitogeográficas argentinas. In: Kugel WF (ed) Enciclopedia Argentina de agricultura y jardinería II, 2nd edn. ACME, Buenos Aires
Calviño A (2006) Evaluación del éxito reproductivo de Caesalpinia gilliesii (Fabaceae) en función de la densidad a distintas escalas. Ph.D. Dissertation, Universidad Nacional de Córdoba. Argentina
Calviño A, Carrizo García C (2005) Sexual dimorphism and gynoecium size variation in the andromonoecious shrub Caesalpinia gilliesii. Plant Biol 7:195–202. doi:10.1055/s-2005-837576
Cao G-X, Kudo G (2008) Size-dependent sex allocation in a monocarpic perennial herb, Cardiocrinum cordatum (Liliaceae). Plant Ecol 194:99–107. doi:10.1007/s11258-007-9277-x
Cocucci AA, Galetto L, Sersic A (1992) El síndrome floral de Caesalpinia gilliesii (Fabaceae-Caesalpinioideae). Darwiniana 31:111–135
de Jong TJ, Klinkhamer PGL (1994) Plant size and reproductive success through female and male function. J Ecol 82:399–402
de Jong TJ, Klinkhamer PGL (2005) Evolutionary ecology of plant reproductive strategies. Cambridge University Press, New York, pp 156–183
Diggle PK (1994) The expression of andromonoecy in Solanum hirtum (Solanaceae): phenotypic plasticity and ontogenetic contingency. Am J Bot 81:1354–1365
Dorken ME, Barrett SCH (2003) Gender plasticity in Sagittaria sagittifolia (Alismataceae), a monoecious aquatic species. Plant Syst Evol 237:99–106. doi:10.1007/s00606-002-0243-8
Elle E, Meagher TR (2000) Sex allocation and reproductive success in the andromonoecious perennial Solanum carolinense (Solanaceae). II. Paternity and functional gender. Am Nat 156:622–636
Emms SK (1993) Andromonoecy in Zigadenus paniculatus (Liliaceae): spatial and temporal patterns of sex allocation. Am J Bot 80:914–923
Faliński JB (1998) Androgyny of individuals and polygamy in populations of Salix myrsinifolia Salisb. in the south-western part of its geographical range (NE-Poland). Perspect Plant Ecol Evol Syst 1(2):238–266
Gibbs PE, Lewis GP, Lughadha EN (1999) Fruit-set induced changes in the sex of flowers in Caesalpinia calycina (Leguminosae). Plant Biol 1:665–669
Griffin SR, Barrett SCH (2002) Factors affecting low seed: ovule ratios in a spring woodland herb, Trillium grandiflorum (Melanthiaceae). Int J Plant Sci 163:581–590
Guitián J, Medrano M, Oti J (2004) Variation in floral sex allocation in Polygonatum odoratum (Liliaceae). Ann Bot 94:433–440. doi:10.1093/aob/mch163
Herrera CM (1991) Dissecting factors responsible for individual variation in plant fecundity. Ecology 72:1436–1448
Ishii HS (2004) Increase of male reproductive components with size in an animal-pollinated perfect, Narthecium asiaticum (Liliaceae). Funct Ecol 18:130–137
Jausoro M, Galetto L (2001) Producción de flores y frutos en una especie andromonoica: Caesalpinia gilliesii (Fabaceae). Kurtziana 29:15–25
Kearns CA, Inouye DW (1993) Environmental measurements for pollination studies. University Press of Colorado, Niwot, CO
Klinkhamer PGL, de Jong TJ (1997) Size dependent allocation to male and female reproduction. In: Bazzaz FA, Grace J (eds) Plant resource allocation. Academic Press, London, pp 211–229
Klinkhamer PGL, de Jong TJ, Metz H (1997) Sex and size in cosexual plants. Trends Ecol Evol 12:260–265
Korpelainen H (1998) Labile sex expression in plants. Biol Rev 73:157–180
Krupnick GA, Weis AE (1998) Floral herbivore effect on the sex expression of an andromonoecious plant, Isomeris arborea (Capparaceae). Plant Ecol 134:151–162
Kunin WE (1992) Density and reproductive success in wild populations of Diplotaxis erucoides (Brassicaceae). Oecologia 91:129–133
Liao W-J, Zhang D-Y (2008) Increased maleness at flowering stage and femaleness at fruiting stage with size in an andromonoecious perennial, Veratrum nigrum. J Integr Plant Biol 50:1024–1030. doi:10.1111/j.1744-7909.2008.00691.x
Liao W-J, Song Q-F, Zhang D-Y (2006) Pollen and resource limitation in Veratrum nigrum L. (Liliaceae), an andromonoecious herb. J Integr Plant Biol 48:1401–1408. doi:10.1111/j.1672-9072.2006.00383.x
Liu F, Yue X-L, Chen J-M, Wang Q-F (2008) Gender modification in a monoecious species Sagittaria potamogetifolia (Alismataceae). Plant Ecol 199:217–223. doi:10.1007/s11258-008-9426-x
Lloyd DG (1980a) Sexual strategies in plants I. An hypothesis of serial adjustment of maternal investment during one reproductive session. New Phytol 86:69–79
Lloyd DG (1980b) Sexual strategies in plants III. A quantitative method for describing gender of plants. N Z J Bot 18:103–108
Luti R, Solis M, Galera FM, Müller N, Berzal N, Nores M, Herrera M, Barrera JC (1979) Vegetación. In: Vázquez JR, Roque M (eds) Geografía Física de Córdoba. Editorial Boldt, Buenos Aires, pp 297–368
Méndez M, Karlsson PS (2004) Between-population variation in size-dependent reproduction and reproductive allocation in Pinguicula vulgaris (Lentibulariaceae) and its environmental correlates. Oikos 104:59–70
Moré M, Sérsic AN, Cocucci AA (2006) Specialized use of pollen vectors by Caesalpinia gilliesii, a legume species with brush-type flowers. Biol J Linn Soc 88:579–592
Mustajärvi K, Siikamäki P, Rytkönen S, Lammi A (2001) Consequences of plant population size and density for plant-pollinator interactions and plant performance. J Ecol 89:80–87
Pannell J (1997) Variation in sex ratios and sex allocation in androdioecious Mercurialis annua. J Ecol 85:57–69
Roll J, Mitchell RJ, Cabin RJ, Marshall DL (1997) Reproductive success increases with local density of conspecifics in a desert mustard (Lesquerella fendleri). Conserv Biol 11:738–746
Sarkissian TS, Barrett SCH, Harder LD (2001) Gender variation in Sagittaria latifolia (Alismataceae): is size all that matters? Ecology 82:360–373
Schlessman MA, Graceffa LM (2002) Protogyny, pollination, and sex expression in the andromonoecious Pseudocymopterus montanus (Apiaceae, Apioideae). Int J Plant Sci 163:409–417
Silvertown JW, Lovett Doust J (1993) Introduction to plant population biology. Blackwell, Oxford, pp 51–71
Solomon BP (1985) Environmentally influenced changes in sex expression in an andromonoecious plant. Ecology 66:1321–1332
Ulibarri EA (1997) Flora Fanerogámica Argentina. Fabaceae-parte I. ProFlora 32:6–13
Vallejo-Marín M, Rausher MV (2007) Selection through female fitness helps to explain the maintenance of male flowers. Am Nat 169:563–568
Weiner J (1988) The influence of competition on plant reproduction. In: Lovett Doust J, Lovett Doust L (eds) Plant reproductive ecology: patterns and strategies. Oxford University Press, New York, pp 228–245
Acknowledgments
We thank L. Ashworth, J. Astegiano and two anonymous reviewers for helpful suggestions on an earlier version of the manuscript. This study was supported by Ministerio de Ciencia y Técnica de la Provincia de Córdoba, Secretaría de Ciencia y Técnica de la Universidad Nacional de Córdoba, and Agencia de Promoción Científica y Tecnológica de la Nación (FONCyT). AC and LG are researchers of Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Calviño, A., Galetto, L. Variation in sexual expression in relation to plant height and local density in the andromonoecious shrub Caesalpinia gilliesii (Fabaceae). Plant Ecol 209, 37–45 (2010). https://doi.org/10.1007/s11258-009-9717-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-009-9717-x