Abstract
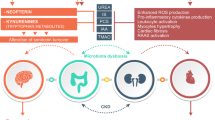
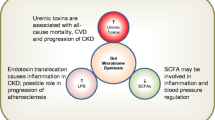
Chronic kidney disease (CKD) is a worldwide health problem, because it is one of the most common complications of metabolic diseases including obesity and type 2 diabetes. Patients with CKD also develop other comorbidities, such as hypertension, hyperlipidemias, liver and cardiovascular diseases, gastrointestinal problems, and cognitive deterioration, which worsens their health. Therapy includes reducing comorbidities or using replacement therapy, such as peritoneal dialysis, hemodialysis, and organ transplant. Health care systems are searching for alternative treatments for CKD patients to mitigate or retard their progression. One new topic is the study of uremic toxins (UT), which are excessively produced during CKD as products of food metabolism or as a result of the loss of renal function that have a negative impact on the kidneys and other organs. High urea concentrations significantly modify the microbiota in the gut also, cause a decrease in bacterial strains that produce anti-inflammatory and fuel molecules and an increase in bacterial strains that can metabolize urea, but also produce UT, including indoxyl sulfate and p-cresol sulfate. UT activates several cellular processes that induce oxidative environments, inflammation, proliferation, fibrosis development, and apoptosis; these processes mainly occur in the gut, heart, and kidney. The study of the microbiota during CKD allowed for the implementation of therapy schemes to try to reduce the circulating concentrations of UT and reduce the damage. The objective of this review is to show an overview to know the main UT produced in end-stage renal disease patients, and how prebiotics and probiotics intervention acts as a helpful tool in CKD treatment.


Similar content being viewed by others
References
Levey AS, Coresh J (2012) Chronic kidney disease. Lancet 379(9811):165–180. https://doi.org/10.1016/s0140-6736(11)60178-5
Stevens PE, Levin A (2013) Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Internal Med 158:825–830
Karbach SH, Schonfelder T, Brandao I, Wilms E, Hormann N, Jackel S, Schuler R, Finger S, Knorr M, Lagrange J, Brandt M, Waisman A, Kossmann S, Schafer K, Munzel T, Reinhardt C, Wenzel P (2016) Gut microbiota promote angiotensin II-induced arterial hypertension and vascular dysfunction. J Am Heart Assoc. https://doi.org/10.1161/jaha.116.003698
Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, Zhao M-h, Lv J, Garg AX, Knight J, Rodgers A, Gallagher M, Kotwal S, Cass A, Perkovic V (2015) Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet 385(9981):1975–1982. https://doi.org/10.1016/s0140-6736(14)61601-9
Levin A, Stevens PE, Bilous RW, Coresh J, De Francisco ALM, De Jong PE, Griffith KE, Hemmelgarn BR, Iseki K, Lamb EJ, Levey AS, Riella MC, Shlipak MG, Wang H, White CT, Winearls CG (2013) Kidney disease: improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3(1):1–150. https://doi.org/10.1038/kisup.2012.73
Szummer K, Evans M, Carrero JJ, Alehagen U, Dahlstrom U, Benson L, Lund LH (2017) Comparison of the chronic kidney disease epidemiology collaboration, the modification of diet in renal disease study and the cockcroft-gault equation in patients with heart failure. Open Heart 4(2):e000568. https://doi.org/10.1136/openhrt-2016-000568
Evenepoel P, Poesen R, Meijers B (2017) The gut-kidney axis. Pediatr Nephrol 32(11):2005–2014. https://doi.org/10.1007/s00467-016-3527-x
Castillo-Rodriguez E, Fernandez-Prado R, Esteras R, Perez-Gomez MV, Gracia-Iguacel C, Fernandez-Fernandez B, Kanbay M, Tejedor A, Lazaro A, Ruiz-Ortega M, Gonzalez-Parra E, Sanz AB, Ortiz A, Sanchez-Nino MD (2018) Impact of altered intestinal microbiota on chronic kidney disease progression. Toxins (Basel). https://doi.org/10.3390/toxins10070300
Cosola C, Rocchetti MT, Cupisti A, Gesualdo L (2018) Microbiota metabolites: pivotal players of cardiovascular damage in chronic kidney disease. Pharmacol Res 130:132–142. https://doi.org/10.1016/j.phrs.2018.03.003
Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D (2015) Role of the normal gut microbiota. World J Gastroenterol 21(29):8787–8803. https://doi.org/10.3748/wjg.v21.i29.8787
Mishima E, Fukuda S, Mukawa C, Yuri A, Kanemitsu Y, Matsumoto Y, Akiyama Y, Fukuda NN, Tsukamoto H, Asaji K, Shima H, Kikuchi K, Suzuki C, Suzuki T, Tomioka Y, Soga T, Ito S, Abe T (2017) Evaluation of the impact of gut microbiota on uremic solute accumulation by a CE-TOFMS-based metabolomics approach. Kidney Int 92(3):634–645. https://doi.org/10.1016/j.kint.2017.02.011
Cremon C, Barbaro MR, Ventura M, Barbara G (2018) Pre- and probiotic overview. Curr Opin Pharmacol 43:87–92. https://doi.org/10.1016/j.coph.2018.08.010
Afsar B, Vaziri ND, Aslan G, Tarim K, Kanbay M (2016) Gut hormones and gut microbiota: implications for kidney function and hypertension. J Am Soc Hypertens 10(12):954–961. https://doi.org/10.1016/j.jash.2016.10.007
Al Khodor S, Reichert B, Shatat IF (2017) The microbiome and blood pressure: can microbes regulate our blood pressure? Front Pediatrics. https://doi.org/10.3389/fped.2017.00138
Aron-Wisnewsky J, Clement K (2016) The gut microbiome, diet, and links to cardiometabolic and chronic disorders. Nat Rev Nephrol 12(3):169–181. https://doi.org/10.1038/nrneph.2015.191
Kikuchi M, Ueno M, Itoh Y, Suda W, Hattori M (2017) Uremic toxin-producing gut microbiota in rats with chronic kidney disease. Nephron 135(1):51–60. https://doi.org/10.1159/000450619
Lee JR, Magruder M, Zhang L, Westblade LF, Satlin MJ, Robertson A, Edusei E, Crawford C, Ling L, Taur Y, Schluter J, Lubetzky M, Dadhania D, Pamer E, Suthanthiran M (2018) Gut microbiota dysbiosis and diarrhea in kidney transplant recipients. Am J Transplant. https://doi.org/10.1111/ajt.14974
Rosas-Villegas A, Sanchez-Tapia M, Avila-Nava A, Ramirez V, Tovar AR, Torres N (2017) Differential effect of sucrose and fructose in combination with a high fat diet on intestinal microbiota and kidney oxidative stress. Nutrients. https://doi.org/10.3390/nu9040393
Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, Verbeke K, Reid G (2017) Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol 14(8):491–502. https://doi.org/10.1038/nrgastro.2017.75
Cresci GA, Bawden E (2015) Gut microbiome: what we do and don’t know. Nutr Clin Pract 30(6):734–746. https://doi.org/10.1177/0884533615609899
Obrenovich MEM (2018) Leaky gut, leaky brain? Microorganisms. https://doi.org/10.3390/microorganisms6040107
Gaulke CA, Sharpton TJ (2018) The influence of ethnicity and geography on human gut microbiome composition. Nat Med. https://doi.org/10.1038/s41591-018-0210-8
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME (2014) Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11(8):506–514. https://doi.org/10.1038/nrgastro.2014.66
Borges NA, Stenvinkel P, Bergman P, Qureshi AR, Lindholm B, Moraes C, Stockler-Pinto MB, Mafra D (2018) Effects of probiotic supplementation on trimethylamine-N-oxide plasma levels in hemodialysis patients: a pilot study. Probiotics Antimicrob Proteins. https://doi.org/10.1007/s12602-018-9411-1
Duranton F, Cohen G, De Smet R, Rodriguez M, Jankowski J, Vanholder R, Argiles A, European Uremic Toxin Work G (2012) Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol 23(7):1258–1270. https://doi.org/10.1681/asn.2011121175
Meijers B, Farre R, Dejongh S, Vicario M, Evenepoel P (2018) Intestinal barrier function in chronic kidney disease. Toxins (Basel). https://doi.org/10.3390/toxins10070298
Mydlik M, Derzsiova K (2008) Oxalic acid as a uremic toxin. J Ren Nutr 18(1):33–39. https://doi.org/10.1053/j.jrn.2007.10.008
Cosola C, Rocchetti MT, Sabatino A, Fiaccadori E, Di Iorio BR, Gesualdo L (2018) Microbiota issue in CKD: how promising are gut-targeted approaches? J Nephrol. https://doi.org/10.1007/s40620-018-0516-0
Han H, Zhu J, Zhu Z, Ni J, Du R, Dai Y, Chen Y, Wu Z, Lu L, Zhang R (2015) p-Cresyl sulfate aggravates cardiac dysfunction associated with chronic kidney disease by enhancing apoptosis of cardiomyocytes. J Am Heart Assoc 4(6):e001852. https://doi.org/10.1161/jaha.115.001852
Sun CY, Chang SC, Wu MS (2012) Suppression of Klotho expression by protein-bound uremic toxins is associated with increased DNA methyltransferase expression and DNA hypermethylation. Kidney Int 81(7):640–650. https://doi.org/10.1038/ki.2011.445
Borges NA, Barros AF, Nakao LS, Dolenga CJ, Fouque D, Mafra D (2016) Protein-bound uremic toxins from gut microbiota and inflammatory markers in chronic kidney disease. J Ren Nutr 26(6):396–400. https://doi.org/10.1053/j.jrn.2016.07.005
Kim HY, Yoo TH, Hwang Y, Lee GH, Kim B, Jang J, Yu HT, Kim MC, Cho JY, Lee CJ, Kim HC, Park S, Lee WW (2017) Indoxyl sulfate (IS)-mediated immune dysfunction provokes endothelial damage in patients with end-stage renal disease (ESRD). Sci Rep 7(1):3057. https://doi.org/10.1038/s41598-017-03130-z
Matsumoto T, Takayanagi K, Kojima M, Taguchi K, Kobayashi T (2019) Acute exposure to indoxyl sulfate impairs endothelium-dependent vasorelaxation in rat aorta. Int J Mol Sci. https://doi.org/10.3390/ijms20020338
Liu S, Wang BH, Kompa AR, Lekawanvijit S, Krum H (2012) Antagonists of organic anion transporters 1 and 3 ameliorate adverse cardiac remodelling induced by uremic toxin indoxyl sulfate. Int J Cardiol 158(3):457–458. https://doi.org/10.1016/j.ijcard.2012.05.022
Sato B, Yoshikawa D, Ishii H, Suzuki S, Inoue Y, Takeshita K, Tanaka M, Kumagai S, Matsumoto M, Okumura S, Hayashi M, Matsubara T, Niwa T, Murohara T (2013) Relation of plasma indoxyl sulfate levels and estimated glomerular filtration rate to left ventricular diastolic dysfunction. Am J Cardiol 111(5):712–716. https://doi.org/10.1016/j.amjcard.2012.11.025
Shiotsu Y, Neckers LM, Wortman I, An WG, Schulte TW, Soga S, Murakata C, Tamaoki T, Akinaga S (2000) Novel oxime derivatives of radicicol induce erythroid differentiation associated with preferential G(1) phase accumulation against chronic myelogenous leukemia cells through destabilization of Bcr-Abl with Hsp90 complex. Blood 96(6):2284–2291
Hai X, Landeras V, Dobre MA, DeOreo P, Meyer TW, Hostetter TH (2015) Mechanism of prominent trimethylamine oxide (TMAO) accumulation in hemodialysis patients. PLoS One 10(12):e0143731. https://doi.org/10.1371/journal.pone.0143731
Tang WH, Kitai T, Hazen SL (2017) Gut microbiota in cardiovascular health and disease. Circ Res 120(7):1183–1196. https://doi.org/10.1161/circresaha.117.309715
Tang WH, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, Li XS, Levison BS, Hazen SL (2015) Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res 116(3):448–455. https://doi.org/10.1161/circresaha.116.305360
Glassock RJ (2008) Uremic toxins: what are they? An integrated overview of pathobiology and classification. J Ren Nutr 18(1):2–6. https://doi.org/10.1053/j.jrn.2007.10.003
Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Johansen K, Kasiske B, Kutner N, Liu J, St Peter W, Guo H, Gustafson S, Heubner B, Lamb K, Li S, Li S, Peng Y, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Thompson B, Wang C, Weinhandl E, Zaun D, Arko C, Chen SC, Daniels F, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers P, Agodoa L (2012) ‘United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis 59(1 Suppl 1):A7, e1-420. https://doi.org/10.1053/j.ajkd.2011.11.015
Ermer T, Eckardt KU, Aronson PS, Knauf F (2016) Oxalate, inflammasome, and progression of kidney disease. Curr Opin Nephrol Hypertens 25(4):363–371. https://doi.org/10.1097/mnh.0000000000000229
Turkmen K, Erdur FM (2015) The relationship between colonization of Oxalobacter formigenes serum oxalic acid and endothelial dysfunction in hemodialysis patients: from impaired colon to impaired endothelium. Med Hypotheses 84(3):273–275. https://doi.org/10.1016/j.mehy.2015.01.010
Gulhan B, Turkmen K, Aydin M, Gunay M, Cikman A, Kara M (2015) The relationship between serum oxalic acid, central hemodynamic parameters and colonization by oxalobacter formigenes in hemodialysis patients. Cardiorenal Med 5(3):164–174. https://doi.org/10.1159/000381219
Mulay SR, Eberhard JN, Pfann V, Marschner JA, Darisipudi MN, Daniel C, Romoli S, Desai J, Grigorescu M, Kumar SV, Rathkolb B, Wolf E, Hrabe de Angelis M, Bauerle T, Dietel B, Wagner CA, Amann K, Eckardt KU, Aronson PS, Anders HJ, Knauf F (2016) Oxalate-induced chronic kidney disease with its uremic and cardiovascular complications in C57BL/6 mice. Am J Physiol Renal Physiol 310(8):F785–F795. https://doi.org/10.1152/ajprenal.00488.2015
Vanholder R, De Smet R, Glorieux G, Argiles A, Baurmeister U, Brunet P, Clark W, Cohen G, De Deyn PP, Deppisch R, Descamps-Latscha B, Henle T, Jorres A, Lemke HD, Massy ZA, Passlick-Deetjen J, Rodriguez M, Stegmayr B, Stenvinkel P, Tetta C, Wanner C, Zidek W, European Uremic Toxin Work G (2003) Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int 63(5):1934–1943. https://doi.org/10.1046/j.1523-1755.2003.00924.x
Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ, Ni Z, Nguyen TH, Andersen GL (2013) Chronic kidney disease alters intestinal microbial flora. Kidney Int 83(2):308–315. https://doi.org/10.1038/ki.2012.345
Wong J, Piceno YM, DeSantis TZ, Pahl M, Andersen GL, Vaziri ND (2014) Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am J Nephrol 39(3):230–237. https://doi.org/10.1159/000360010
Yacoub R, Wyatt CM (2017) Manipulating the gut microbiome to decrease uremic toxins. Kidney Int 91(3):521–523. https://doi.org/10.1016/j.kint.2017.01.003
Yeh YC, Huang MF, Liang SS, Hwang SJ, Tsai JC, Liu TL, Wu PH, Yang YH, Kuo KC, Kuo MC, Chen CS (2016) Indoxyl sulfate, not p-cresyl sulfate, is associated with cognitive impairment in early-stage chronic kidney disease. Neurotoxicology 53:148–152. https://doi.org/10.1016/j.neuro.2016.01.006
Suchy-Dicey AM, Laha T, Hoofnagle A, Newitt R, Sirich TL, Meyer TW, Thummel KE, Yanez ND, Himmelfarb J, Weiss NS, Kestenbaum BR (2016) Tubular secretion in CKD. J Am Soc Nephrol 27(7):2148–2155. https://doi.org/10.1681/asn.2014121193
Lu LF, Tang WH, Hsu CC, Tsai IT, Hung WC, Yu TH, Wu CC, Chung FM, Lu YC, Lee YJ, Wang CP (2016) Associations among chronic kidney disease, high total p-cresylsulfate and left ventricular systolic dysfunction. Clin Chim Acta 457:63–68. https://doi.org/10.1016/j.cca.2016.03.012
Jiang S, Xie S, Lv D, Wang P, He H, Zhang T, Zhou Y, Lin Q, Zhou H, Jiang J, Nie J, Hou F, Chen Y (2017) Alteration of the gut microbiota in Chinese population with chronic kidney disease. Sci Rep 7(1):2870. https://doi.org/10.1038/s41598-017-02989-2
Xu KY, Xia GH, Lu JQ, Chen MX, Zhen X, Wang S, You C, Nie J, Zhou HW, Yin J (2017) Impaired renal function and dysbiosis of gut microbiota contribute to increased trimethylamine-N-oxide in chronic kidney disease patients. Sci Rep 7(1):1445. https://doi.org/10.1038/s41598-017-01387-y
Banerjee T, Meyer TW, Shafi T, Hostetter TH, Melamed M, Zhu Y, Powe NR (2017) Free and total p-cresol sulfate levels and infectious hospitalizations in hemodialysis patients in CHOICE and HEMO. Medicine (Baltimore) 96(6):e5799. https://doi.org/10.1097/md.0000000000005799
WHO, DCO, WHD, 2013.2 (2013) Información general sobre la HIPERTENSIÓN en el mundo. WHO, Geneva, pp 1–40
Kelly JP, Curhan GC, Cave DR, Anderson TE, Kaufman DW (2011) Factors related to colonization with Oxalobacter formigenes in U.S. adults. J Endourol 25(4):673–679. https://doi.org/10.1089/end.2010.0462
Chilton SN, Burton JP, Reid G (2015) Inclusion of fermented foods in food guides around the world. Nutrients 7(1):390–404. https://doi.org/10.3390/nu7010390
Sluijs I, Forouhi NG, Beulens JW, van der Schouw YT, Agnoli C, Arriola L, Balkau B, Barricarte A, Boeing H, Bueno-de-Mesquita HB, Clavel-Chapelon F, Crowe FL, de Lauzon-Guillain B, Drogan D, Franks PW, Gavrila D, Gonzalez C, Halkjaer J, Kaaks R, Moskal A, Nilsson P, Overvad K, Palli D, Panico S, Quiros JR, Ricceri F, Rinaldi S, Rolandsson O, Sacerdote C, Sanchez MJ, Slimani N, Spijkerman AM, Teucher B, Tjonneland A, Tormo MJ, Tumino R, van der A DL, Sharp SJ, Langenberg C, Feskens EJ, Riboli E, Wareham NJ, InterAct C (2012) The amount and type of dairy product intake and incident type 2 diabetes: results from the EPIC-InterAct Study. Am J Clin Nutr 96(2):382–390. https://doi.org/10.3945/ajcn.111.021907
Lau E, Neves JS, Ferreira-Magalhaes M, Carvalho D, Freitas P (2019) Probiotic ingestion, obesity, and metabolic-related disorders: results from NHANES, 1999-2014. Nutrients. https://doi.org/10.3390/nu11071482
Barengolts E, Smith ED, Reutrakul S, Tonucci L, Anothaisintawee T (2019) The effect of probiotic yogurt on glycemic control in type 2 diabetes or obesity: a meta-analysis of nine randomized controlled trials. Nutrients. https://doi.org/10.3390/nu11030671
Punaro GR, Maciel FR, Rodrigues AM, Rogero MM, Bogsan CS, Oliveira MN, Ihara SS, Araujo SR, Sanches TR, Andrade LC, Higa EM (2014) Kefir administration reduced progression of renal injury in STZ-diabetic rats by lowering oxidative stress. Nitric Oxide 37:53–60. https://doi.org/10.1016/j.niox.2013.12.012
Sari EK, Bakir B, Aydin BD, Sozmen M (2014) The effects of kefir, koumiss, yogurt and commercial probiotic formulations on PPARalpha and PPAR-beta/delta expressions in mouse kidney. Biotech Histochem 89(4):287–295. https://doi.org/10.3109/10520295.2013.844274
Dumas AA, Lapointe A, Dugrenier M, Provencher V, Lamarche B, Desroches S (2017) A systematic review of the effect of yogurt consumption on chronic diseases risk markers in adults. Eur J Nutr 56(4):1375–1392. https://doi.org/10.1007/s00394-016-1341-7
Sirich TL, Plummer NS, Gardner CD, Hostetter TH, Meyer TW (2014) Effect of increasing dietary fiber on plasma levels of colon-derived solutes in hemodialysis patients. Clin J Am Soc Nephrol 9(9):1603–1610. https://doi.org/10.2215/cjn.00490114
Vaziri ND, Liu SM, Lau WL, Khazaeli M, Nazertehrani S, Farzaneh SH, Kieffer DA, Adams SH, Martin RJ (2014) High amylose resistant starch diet ameliorates oxidative stress, inflammation, and progression of chronic kidney disease. PLoS One 9(12):e114881. https://doi.org/10.1371/journal.pone.0114881
Kieffer DA, Piccolo BD, Vaziri ND, Liu S, Lau WL, Khazaeli M, Nazertehrani S, Moore ME, Marco ML, Martin RJ, Adams SH (2016) Resistant starch alters gut microbiome and metabolomic profiles concurrent with amelioration of chronic kidney disease in rats. Am J Physiol Renal Physiol 310(9):F857–F871. https://doi.org/10.1152/ajprenal.00513.2015
Laffin MR, Tayebi Khosroshahi H, Park H, Laffin LJ, Madsen K, Kafil HS, Abedi B, Shiralizadeh S, Vaziri ND (2019) Amylose resistant starch (HAM-RS2) supplementation increases the proportion of Faecalibacterium bacteria in end-stage renal disease patients: microbial analysis from a randomized placebo-controlled trial. Hemodial Int 23(3):343–347. https://doi.org/10.1111/hdi.12753
Yoshifuji A, Wakino S, Irie J, Matsui A, Hasegawa K, Tokuyama H, Hayashi K, Itoh H (2018) Oral adsorbent AST-120 ameliorates gut environment and protects against the progression of renal impairment in CKD rats. Clin Exp Nephrol 22(5):1069–1078. https://doi.org/10.1007/s10157-018-1577-z
Yamamoto S, Zuo Y, Ma J, Yancey PG, Hunley TE, Motojima M, Fogo AB, Linton MF, Fazio S, Ichikawa I, Kon V (2011) Oral activated charcoal adsorbent (AST-120) ameliorates extent and instability of atherosclerosis accelerated by kidney disease in apolipoprotein E-deficient mice. Nephrol Dial Transplant 26(8):2491–2497. https://doi.org/10.1093/ndt/gfq759
Sato E, Saigusa D, Mishima E, Uchida T, Miura D, Morikawa-Ichinose T, Kisu K, Sekimoto A, Saito R, Oe Y, Matsumoto Y, Tomioka Y, Mori T, Takahashi N, Sato H, Abe T, Niwa T, Ito S (2017) Impact of the oral adsorbent AST-120 on organ-specific accumulation of uremic toxins: LC-MS/MS and MS imaging techniques. Toxins (Basel). https://doi.org/10.3390/toxins10010019
Ali BH, Inuwa I, Al Za’abi M, Al Bahlani S, Al Issaei H, Ramkumar A, Madanagopal T, Nemmar A, Malheiros DM, Zatz R (2014) Renal and myocardial histopathology and morphometry in rats with adenine—induced chronic renal failure: influence of gum acacia. Cell Physiol Biochem 34(3):818–828. https://doi.org/10.1159/000363045
Al Za’abi M, Al Busaidi M, Yasin J, Schupp N, Nemmar A, Ali BH (2015) Development of a new model for the induction of chronic kidney disease via intraperitoneal adenine administration, and the effect of treatment with gum acacia thereon. Am J Transl Res 7(1):28–38
Al Suleimani YM, Al Za’abi M, Ramkumar A, Al Mahruqi AS, Tageldin MH, Nemmar A, Ali BH (2015) Influence of treatment with gum acacia on renal vascular responses in a rat model of chronic kidney disease. Eur Rev Med Pharmacol Sci 19(3):498–506
Al Za’abi M, Al Salam S, Al Suleimani Y, Manoj P, Nemmar A, Ali BH (2018) Gum acacia improves renal function and ameliorates systemic inflammation, oxidative and nitrosative stress in streptozotocin-induced diabetes in rats with adenine-induced chronic kidney disease. Cell Physiol Biochem 45(6):2293–2304. https://doi.org/10.1159/000488176
Feng YL, Cao G, Chen DQ, Vaziri ND, Chen L, Zhang J, Wang M, Guo Y, Zhao YY (2019) Microbiome-metabolomics reveals gut microbiota associated with glycine-conjugated metabolites and polyamine metabolism in chronic kidney disease. Cell Mol Life Sci. https://doi.org/10.1007/s00018-019-03155-9
Niwa T, Tsukushi S, Ise M, Miyazaki T, Tsubakihara Y, Owada A, Shiigai T (1997) Indoxyl sulfate and progression of renal failure: effects of a low-protein diet and oral sorbent on indoxyl sulfate production in uremic rats and undialyzed uremic patients. Miner Electrolyte Metab 23(3–6):179–184
Menon V, Kopple JD, Wang X, Beck GJ, Collins AJ, Kusek JW, Greene T, Levey AS, Sarnak MJ (2009) Effect of a very low-protein diet on outcomes: long-term follow-up of the Modification of Diet in Renal Disease (MDRD) Study. Am J Kidney Dis 53(2):208–217. https://doi.org/10.1053/j.ajkd.2008.08.009
Elamin S, Alkhawaja MJ, Bukhamsin AY, Idris MAS, Abdelrahman MM, Abutaleb NK, Housawi AA (2017) Gum arabic reduces C-reactive protein in chronic kidney disease patients without affecting urea or indoxyl sulfate levels. Int J Nephrol 2017:9501470. https://doi.org/10.1155/2017/9501470
Esgalhado M, Kemp JA, Azevedo R, Paiva BR, Stockler-Pinto MB, Dolenga CJ, Borges NA, Nakao LS, Mafra D (2018) Could resistant starch supplementation improve inflammatory and oxidative stress biomarkers and uremic toxins levels in hemodialysis patients? A pilot randomized controlled trial. Food Funct 9(12):6508–6516. https://doi.org/10.1039/c8fo01876f
Pavan M (2016) Influence of prebiotic and probiotic supplementation on the progression of chronic kidney disease. Minerva Urol Nefrol 68(2):222–226
Rossi M, Johnson DW, Morrison M, Pascoe E, Coombes JS, Forbes JM, McWhinney BC, Ungerer JPJ, Dimeski G, Campbell KL (2014) SYNbiotics Easing Renal failure by improving Gut microbiologY (SYNERGY): a protocol of placebo-controlled randomised cross-over trial. BMC Nephrol 15:106
Simeoni M, Citraro ML, Deodato F, Provenzano M, Capria M, Comi A, Libri E, Andreucci M, Puja A, Abenavoli L, Cocchi M, Fuiano G (2018) An open-label, randomized, placebo-controlled study on the effectiveness of a novel probiotics administration protocol (ProbiotiCKD) in patients with mild renal insufficiency (stage 3a of CKD). Eur J Nutr. https://doi.org/10.1007/s00394-018-1785-z
Thongprayoon C, Hatch ST, Kaewput W, Sharma K, Ungprasert P, Wijarnpreecha K, D’Costa M, Mao MA, Cheungpasitporn W (2018) The effects of probiotics on renal function and uremic toxins in patients with chronic kidney disease; a meta-analysis of randomized controlled trials. J Nephropathol 7(3):106–114. https://doi.org/10.15171/jnp.2018.25
Thongprayoon C, Kaewput W, Hatch ST, Bathini T, Sharma K, Wijarnpreecha K, Ungprasert P, D’Costa M, Mao MA, Cheungpasitporn W (2019) Effects of probiotics on inflammation and uremic toxins among patients on dialysis: a systematic review and meta-analysis. Dig Dis Sci 64(2):469–479. https://doi.org/10.1007/s10620-018-5243-9
Jia L, Jia Q, Yang J, Jia R, Zhang H (2018) Efficacy of probiotics supplementation on chronic kidney disease: a systematic review and meta-analysis. Kidney Blood Press Res 43(5):1623–1635. https://doi.org/10.1159/000494677
Tao S, Tao S, Cheng Y, Liu J, Ma L, Fu P (2018) Effects of probiotic supplements on the progression of chronic kidney disease: a meta-analysis. Nephrology (Carlton). https://doi.org/10.1111/nep.13549
Borges NA, Carmo FL, Stockler-Pinto MB, de Brito JS, Dolenga CJ, Ferreira DC, Nakao LS, Rosado A, Fouque D, Mafra D (2018) Probiotic supplementation in chronic kidney disease: a double-blind, randomized. Placebo-controlled Trial. J Ren Nutr 28(1):28–36. https://doi.org/10.1053/j.jrn.2017.06.010
de Faria Barros A, Borges NA, Nakao LS, Dolenga CJ, do Carmo FL, de Carvalho Ferreira D, Stenvinkel P, Bergman P, Lindholm B, Mafra D (2018) Effects of probiotic supplementation on inflammatory biomarkers and uremic toxins in non-dialysis chronic kidney patients: a double-blind, randomized, placebo-controlled trial. J Funct Foods 46:378–383. https://doi.org/10.1016/j.jff.2018.05.018
Deltombe O, Van Biesen W, Glorieux G, Massy Z, Dhondt A, Eloot S (2015) Exploring protein binding of uremic toxins in patients with different stages of chronic kidney disease and during hemodialysis. Toxins (Basel) 7(10):3933–3946. https://doi.org/10.3390/toxins7103933
Mutsaers HA, Engelke UF, Wilmer MJ, Wetzels JF, Wevers RA, van den Heuvel LP, Hoenderop JG, Masereeuw R (2013) Optimized metabolomic approach to identify uremic solutes in plasma of stage 3-4 chronic kidney disease patients. PLoS One 8(8):e71199. https://doi.org/10.1371/journal.pone.0071199
Motojima M, Hosokawa A, Yamato H, Muraki T, Yoshioka T (2003) Uremic toxins of organic anions up-regulate PAI-1 expression by induction of NF-kappaB and free radical in proximal tubular cells. Kidney Int 63(5):1671–1680. https://doi.org/10.1046/j.1523-1755.2003.00906.x
Han H, Chen Y, Zhu Z, Su X, Ni J, Du R, Zhang R, Jin W (2016) p-Cresyl sulfate promotes the formation of atherosclerotic lesions and induces plaque instability by targeting vascular smooth muscle cells. Front Med 10(3):320–329. https://doi.org/10.1007/s11684-016-0463-x
Kim SH, Yu MA, Ryu ES, Jang YH, Kang DH (2012) Indoxyl sulfate-induced epithelial-to-mesenchymal transition and apoptosis of renal tubular cells as novel mechanisms of progression of renal disease. Lab Invest 92(4):488–498. https://doi.org/10.1038/labinvest.2011.194
Satoh M, Hayashi H, Watanabe M, Ueda K, Yamato H, Yoshioka T, Motojima M (2003) Uremic toxins overload accelerates renal damage in a rat model of chronic renal failure. Nephron Exp Nephrol 95(3):e111–e118. https://doi.org/10.1159/000074327
Nishiyama K, Aono K, Fujimoto Y, Kuwamura M, Okada T, Tokumoto H, Izawa T, Okano R, Nakajima H, Takeuchi T, Azuma YT (2019) Chronic kidney disease after 5/6 nephrectomy disturbs the intestinal microbiota and alters intestinal motility. J Cell Physiol 234(5):6667–6678. https://doi.org/10.1002/jcp.27408
Miranda Alatriste PV, Urbina Arronte R, Gomez Espinosa CO, Espinosa Cuevas Mde L (2014) Effect of probiotics on human blood urea levels in patients with chronic renal failure. Nutr Hosp 29(3):582–590. https://doi.org/10.3305/nh.2014.29.3.7179
Soleimani A, Zarrati Mojarrad M, Bahmani F, Taghizadeh M, Ramezani M, Tajabadi-Ebrahimi M, Jafari P, Esmaillzadeh A, Asemi Z (2017) Probiotic supplementation in diabetic hemodialysis patients has beneficial metabolic effects. Kidney Int 91(2):435–442. https://doi.org/10.1016/j.kint.2016.09.040
Ramos CI, Armani RG, Canziani MEF, Dalboni MA, Dolenga CJR, Nakao LS, Campbell KL, Cuppari L (2018) Effect of prebiotic (fructooligosaccharide) on uremic toxins of chronic kidney disease patients: a randomized controlled trial. Nephrol Dial Transplant. https://doi.org/10.1093/ndt/gfy171
Haghighat N, Mohammadshahi M, Shayanpour S, Haghighizadeh MH (2018) Effect of synbiotic and probiotic supplementation on serum levels of endothelial cell adhesion molecules in hemodialysis patients: a randomized control study. Probiotics Antimicrob Proteins. https://doi.org/10.1007/s12602-018-9477-9
Cruz-Mora J, Martinez-Hernandez NE, Martin del Campo-Lopez F, Viramontes-Horner D, Vizmanos-Lamotte B, Munoz-Valle JF, Garcia-Garcia G, Parra-Rojas I, Castro-Alarcon N (2014) Effects of a symbiotic on gut microbiota in Mexican patients with end-stage renal disease. J Ren Nutr 24(5):330–335. https://doi.org/10.1053/j.jrn.2014.05.006
Xie LM, Ge YY, Huang X, Zhang YQ, Li JX (2015) Effects of fermentable dietary fiber supplementation on oxidative and inflammatory status in hemodialysis patients. Int J Clin Exp Med 8(1):1363–1369
Ranganathan N, Ranganathan P, Friedman EA, Joseph A, Delano B, Goldfarb DS, Tam P, Rao AV, Anteyi E, Musso CG (2010) Pilot study of probiotic dietary supplementation for promoting healthy kidney function in patients with chronic kidney disease. Adv Ther 27(9):634–647. https://doi.org/10.1007/s12325-010-0059-9
Eidi F, Poor-Reza Gholi F, Ostadrahimi A, Dalili N, Samadian F, Barzegari A (2018) Effect of Lactobacillus Rhamnosus on serum uremic toxins (phenol and P-Cresol) in hemodialysis patients: a double blind randomized clinical trial. Clin Nutr ESPEN 28:158–164. https://doi.org/10.1016/j.clnesp.2018.08.010
Natarajan R, Pechenyak B, Vyas U, Ranganathan P, Weinberg A, Liang P, Mallappallil MC, Norin AJ, Friedman EA, Saggi SJ (2014) Randomized controlled trial of strain-specific probiotic formulation (Renadyl) in dialysis patients. Biomed Res Int 2014:568571. https://doi.org/10.1155/2014/568571
Lopes R, Theodoro JMV, da Silva BP, Queiroz VAV, de Castro Moreira ME, Mantovani HC, Hermsdorff HH, Martino HSD (2019) Synbiotic meal decreases uremic toxins in hemodialysis individuals: a placebo-controlled trial. Food Res Int 116:241–248. https://doi.org/10.1016/j.foodres.2018.08.024
Shariaty Z, Mahmoodi Shan GR, Farajollahi M, Amerian M, Behnam Pour N (2017) The effects of probiotic supplement on hemoglobin in chronic renal failure patients under hemodialysis: a randomized clinical trial. J Res Med Sci 22:74. https://doi.org/10.4103/jrms.jrms_614_16
Khosroshahi HT, Abedi B, Ghojazadeh M, Samadi A, Jouyban A (2019) Effects of fermentable high fiber diet supplementation on gut derived and conventional nitrogenous product in patients on maintenance hemodialysis: a randomized controlled trial. Nutr Metab (Lond) 16:18. https://doi.org/10.1186/s12986-019-0343-x
Yang J, Lim SY, Ko YS, Lee HY, Oh SW, Kim MG, Cho WY, Jo SK (2018) Intestinal barrier disruption and dysregulated mucosal immunity contribute to kidney fibrosis in chronic kidney disease. Nephrol Dial Transplant. https://doi.org/10.1093/ndt/gfy172
Iwashita Y, Ohya M, Yashiro M, Sonou T, Kawakami K, Nakashima Y, Yano T, Iwashita Y, Mima T, Negi S, Kubo K, Tomoda K, Odamaki T, Shigematsu T (2018) Dietary changes involving bifidobacterium longum and other nutrients delays chronic kidney disease progression. Am J Nephrol 47(5):325–332. https://doi.org/10.1159/000488947
Nanto-Hara F, Kanemitsu Y, Fukuda S, Kikuchi K, Asaji K, Saigusa D, Iwasaki T, Ho HJ, Mishima E, Suzuki T, Suzuki C, Tsukimi T, Matsuhashi T, Oikawa Y, Akiyama Y, Kure S, Owada Y, Tomioka Y, Soga T, Ito S, Abe T (2019) The guanylate cyclase C agonist linaclotide ameliorates the gut-cardio-renal axis in an adenine-induced mouse model of chronic kidney disease. Nephrol Dial Transplant. https://doi.org/10.1093/ndt/gfz126
Acknowledgements
This work was supported by Grant no. 155700 from the National Council of Science and Technology (CONACyT) to VR.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Plata, C., Cruz, C., Cervantes, L.G. et al. The gut microbiota and its relationship with chronic kidney disease. Int Urol Nephrol 51, 2209–2226 (2019). https://doi.org/10.1007/s11255-019-02291-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-019-02291-2