Abstract
Purpose
To evaluate the negative predictive value (NPV) of a negative prostate multi-parametric magnetic resonance imaging (mpMRI) in ruling out clinically significant prostate upon 12-core systematic biopsy.
Methods
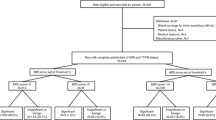
We retrospectively reviewed 114 men evaluated at our institution who underwent systematic 12-core biopsy within 1 year of a negative prostate mpMRI. Clinicopathologic features were evaluated and NPV was calculated for detection of clinically significant (Gleason ≥ 7) cancer. Regression analysis was performed to identify clinical predictors of biopsy outcome.
Results
Overall, 88 (77.2%) patients in our cohort had no cancer detected upon biopsy. The highest pathologic grade was Gleason 6 (3 + 3) in 22 (19.3%) patients, and Gleason ≥ 7 in 4 (3.6%) patients. NPV for detecting Gleason ≥ 7 cancer was 96.5% (95% CI 93.1–99.9%) in the entire negative MRI cohort, 100% in those who were prostate biopsy naïve (n = 20), 100% in those with a prior negative biopsy (n = 53), and 90% in those who have had a previous positive biopsy and on active surveillance (n = 41). Regression analysis identified no predictors of significant cancer in our cohort.
Conclusion
In our cohort of men with no lesions detected on prostate mpMRI, we found very low rates of clinically significant cancer on systematic 12-core biopsy. In the few patients who diagnosed with prostate cancer, the majority had low-risk disease and could remain on active surveillance. Although validation studies and greater sample size is needed before clinical recommendations can be made, our data suggest patients with negative mpMRI evaluated by experienced radiologists may avoid unnecessary prostate biopsy and potential overtreatment.

Similar content being viewed by others
References
Zhao C, Gao G, Fang D et al (2016) The efficiency of multiparametric magnetic resonance imaging (mpMRI) using PI-RADS version 2 in the diagnosis of clinically significant prostate cancer. Clin Imaging 40(5):885–888
Recabal P, Ehdaie B (2015) The role of MRI in active surveillance for men with localized prostate cancer. Curr Opin Urol 25(6):504–509
Rais-Bahrami S, Siddiqui MM, Turkbey B et al (2013) Utility of multiparametric magnetic resonance imaging suspicion levels for detecting prostate cancer. J Urol 190(5):1721–1727
Weinreb JC, Barentsz JO, Choyke PL et al (2016) PI-RADS prostate imaging—reporting and data system: 2015, version 2. Eur Urol 69(1):16–40
Nam RK, Saskin R, Lee Y et al (2010) Increasing hospital admission rates for urological complications after transrectal ultrasound guided prostate biopsy. J Urol 183(3):963–968
Halpern JA, Sedrakyan A, Dinerman B, Hsu WC, Mao J, Hu JC (2016) Indications, utilization and complications following prostate biopsy: New York state analysis. J Urol 197:1020–1025
Ahmed HU, El-Shater Bosaily A, Brown LC et al (2017) Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 389(10071):815–822
Siddiqui MM, Rais-Bahrami S, Turkbey B et al (2015) Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 313(4):390–397
Siddiqui MM, George AK, Rubin R et al (2016) Efficiency of prostate cancer diagnosis by MR/ultrasound fusion-guided biopsy vs standard extended-sextant biopsy for MR-visible lesions. J Natl Cancer Inst 108(9):1–7
Wysock JS, Mendhiratta N, Zattoni F et al (2016) Predictive value of negative 3T multiparametric magnetic resonance imaging of the prostate on 12-core biopsy results. BJU Int 118(4):515–520
Lu AJ, Syed JS, Nguyen KA, et al (2017) Negative multiparametric magnetic resonance imaging of the prostate predicts absence of clinically significant prostate cancer on 12-core template prostate biopsy. Urology 105:118–122
Yerram NK, Volkin D, Turkbey B et al (2012) Low suspicion lesions on multiparametric magnetic resonance imaging predict for the absence of high-risk prostate cancer. BJU Int 110(11 Pt B):E783–E788
Filson C, Margolis D, Huang J et al (2015) MP60-11 should a normal multiparametric MRI preclude prostate biopsy? J Urol 193(4):e742
Boesen L, Norgaard N, Logager V, Thomsen HS (2017) Clinical outcome following low suspicion multiparametric prostate magnetic resonance imaging or benign magnetic resonance imaging guided biopsy to detect prostate cancer: a followup study in men with prior negative transrectal ultrasound guided biopsies. J Urol 310–315
Itatani R, Namimoto T, Atsuji S et al (2014) Negative predictive value of multiparametric MRI for prostate cancer detection: outcome of 5-year follow-up in men with negative findings on initial MRI studies. Eur J Radiol 83(10):1740–1745
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research and the National Institutes of Health (NIH) Medical Research Scholars Program, a public–private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation, The American Association for Dental Research, the Colgate-Palmolive Company, Genentech and alumni of student research programs and other individual supporters via contributions to the Foundation for the National Institutes of Health.
Funding
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research and the National Institutes of Health (NIH) Medical Research Scholars Program, a public–private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation, The American Association for Dental Research, the Colgate-Palmolive Company, Genentech and alumni of student research programs and other individual supporters via contributions to the Foundation for the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author BJW is supported by the Intramural Research Program of the NIH and the NIH Center for Interventional Oncology and NIH Grant # Z1A CL040015-08. NIH and Philips/InVivo Inc have a cooperative Research and Development Agreement. NIH and Philips/InVivo Inc have a patent license agreement and NIH and BJW, BT, PAP, PLC may receive royalties.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Baris Turkbey and Peter A. Pinto have shared senior authorship.
Rights and permissions
About this article
Cite this article
An, J.Y., Sidana, A., Holzman, S.A. et al. Ruling out clinically significant prostate cancer with negative multi-parametric MRI. Int Urol Nephrol 50, 7–12 (2018). https://doi.org/10.1007/s11255-017-1715-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-017-1715-7