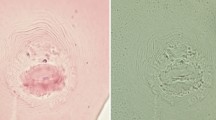
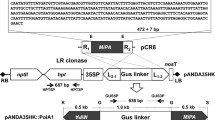
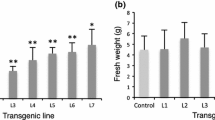
Abstract
Root knot nematodes are serious threats to growth and yield of solaneous crops including tomato. In this study, a binary vector carrying Remusatia vivipara (rvl1) and Sclerotium rolfsii (srl1) lectin genes were introduced independently into Lycopersicon esculentum cv. Pusa Ruby via Agrobacterium tumefaciens for resistance against root knot nematode, Meloidogyne incognita. In total, one hundred and one rvl1 and srl1-transformed plants exhibiting kanamycin resistance were confirmed to carry transgenes as detected by polymerase chain reaction (PCR) with 4.59% transformation efficiency. Genetic analysis of T1 progeny confirmed Mendelian segregation of the introduced genes. Three events each of rvl1 and srl1 transgenic tomato were randomly selected for further confirmation by Southern and TAIL-PCR analyses. All three events of srl1 transgenics showed single copy transgene, whereas two rvl1 transgenic events showed single copy of transgene, while remaining event showed two copies of transgenes. Site of integration obtained for rvl1 and srl1 transgenic events by TAIL-PCR revealed that all the three events of rvl1 and srl1 transgenics differed for their site of integration and insertion sites did not contain any predicted gene. Moreover, expression of the rvl1 and srl1 transgenes was detected by haemagglutination assay in all three events of rvl1 and srl1, but not in non-transgenic tomato plant. Homozygous progenies of these events were grown and inoculated with M. incognita. Development and reproduction of M. incognita was severely affected in transgenic tomato plants expressing RVL1 and SRL1 exhibiting the high levels of resistance compared to non-transgenic plants. Therefore, these transgenic lines demonstrate a promising potential for variety development of tomato lines with enhanced resistance against M. incognita.



Similar content being viewed by others
References
Abad P, Williamson V (2010) Pant nematode interaction: a sophisticated dialogue. Adv Bot Res 53:148–192
Atkinson HJ, Urwin PE, Hussey RS (2009) Plant biotechnology and control. In: Perry RN, Moens M, Starr JL (eds) Root knot nematodes. CABI Publishing, Wallingford, pp 338–362
Aumann J, Wyss U (1987) Lectin binding sites on mobile stages of Heterodera schachtii Schmidt (Nematoda: Heteroderidae). Nematologica 33:410–418
Aumann J, Roberston WM, Wyss U (1991) Lectin binding to cuticle exudates of sedentary Heterodera schachtii (Nematoda: Heteroderidae) second stage juveniles. Rev Nématol 4:113–118
Bertioli DJ, Smoker M, Burrows PR (1999) Nematode-responsive activity of the Cauliflower Mosaic Virus 35S promoter and its subdomains. Mol Plant Microbe Interact 12:189–196
Bhanu-Priya D, Somasekhar J, Prasad S, Kirti PB (2011) Transgenic tobacco plants constitutively expressing Arabidopsis NPR1 show enhanced resistance to root knot nematode, Meloidogyne incognita. BMC Res Notes 4:231–240
Bhat RS, Chandrashekar TM, Basingi SM, Mallesh SB, Lingaraju S (2010) Cloning of Sclerotium rolfsii lectin gene and its nematicidal activity. Curr Sci 98(9):1185–1186
Bhatti DS (1994) Management of Phytonematodes- an Introduction. In: Bhatti DS, Walil RK (eds) Nematode pest management in crops. CBS Pub. and Distributors, Delhi, pp 1–6
Bird AF (1971) Specialised adaptations of nematodes to parasitism. In: Zuckerman BM, Rohde RA, Mai WF (eds) Plant parasitic nematodes, vol 2. Academic Press, New York, pp 35–48
Bleuler-Martínez S, Butschi A, Garbani M, Wälti MA, Wohlschlager T, Potthoff E, Sabotiĉ J, Pohleven J, Lüthy P, Hengartner MO, Aebi M, Künzler M (2011) A lectin-mediated resistance of higher fungi against predators and parasites. Mol Ecol 20(14):3056–3070
Bockenhoff A, Grundler FMW (1994) Studies on the nutrient uptake of the best cyst nematode H. schachtii by in situ microinjection of fluorescent probes into the feeding structures in Arabidopsis thaliana. Parasitology 109:249–254
Bridge J, Page SLJ (1980) Estimation of root-knot nematode infestation levels on roots using a rating chart. Int J Pest Manag 26(3):296–298
Byrd DW, Kirkpatrick T, Barker KR (1983) An improved technique for clearing and staining plant tissues for detection of nematodes. J Nematol 15(1):142–143
Castagnone-Sereno P, Bongiovanni M, Dalmasso A (1992) Differential expression of root-knot nematode resistance genes in tomato and pepper: evidence with Meloidogyne incognita virulent and avirulent near-isogenic lineages. Ann Appl Biol 120:487–492
Chandrashekar TM (2007) Molecular cloning and expression of lectin gene (srl) from Sclerotium rolfsii Sacc. Thesis submitted to University of Agricultural Sciences, Dharwad, India
Cooper DNW, Boulianne RP, Charlton S, Farrell EM, Sucher A, Lu BC (1997) Fungal galectins, sequence and specificity of two isolectins from Coprinus cinereus. J Biol Chem 272(3):1514–1521
Cox KD, Layne DR, Scorza R, Schnabel G (2006) Gastrodia anti-fungal protein from the orchid Gastrodia elata confers disease resistance to root pathogens in transgenic tobacco. Planta 224:1373–1383
Cristofoletti PT, de Sousa FA, Rahbé Y, Terra WR (2006) Characterization of a membrane-bound amino peptidase purified from Acyrthosiphon pisum midgut cells. FEBS J 273:5574–5588
Ehwaeti ME, Elliott MJ, McNicol JM, Phillips MS, Trudgill DL (2000) Modelling nematode population growth and damage. Crop Prot 19:739–745
Ekbote S (2003) Studies on pigeonpea cyst nematode-Heterodera cajani and its interaction with Fusarium udam. Thesis submitted to University of Agricultural Sciences, Dharwad, India
Endo BY (1986) Histology and ultrastructural modification induced by cyst nematodes. In: Lamberti F, Taylor CE (eds) Cyst nematodes. Plenum Press, New York, pp 133–146
Etzler ME (1985) Plant lectins: molecular and biological aspects. Annu Rev Plant Physiol 36(1):209–234
Fitches E, Woodhouse SD, Edwards JP, Gatehouse JA (2001) In vitro and in vivo binding of snowdrop (Galanthus nivalis agglutinin; GNA) and jackbean (Canavalia ensiformis; ConA) lectins within tomato moth (Lacanobia oleracea) larvae; mechanisms of insecticidal action. J Insect Physiol 47(7):777–787
Fukazawa Y, Kagaya K (1997) Molecular bases of adhesion of Candida albicans. Med Mycol 35(2):87–99
Gaofu Q, Shiqing M, Fayin Z, Zhiniu Y, Xiuyun Z (2008) In vitro assessment of plant lectins with anti-pinwood nematode activity. J Invertebr Pathol 98(1):40–45
Goddijn OJM, Lindsey K, Lee FM, Klap JC, Sijmons PC (1993) Differential gene expression in nematode-induced feeding structures of transgenic plants harbouring promoter-gusA fusion constructs. Plant J 4:863–873
Goverse A, Biessheuvel J, Wijers GJ, Gommers FJ, Bakker J, Schots A, Helder J (1998) In Planta monitoring of the activity of two constitutive promoters, CaMV 35S and TR2, in developing feeding cells induced by Globodera rostochiensis using green fluorescent protein in combination with confocal laser scanning microscopy. Physiol Mol Plant Pathol 52:275–284
Hanhinev KJ, Kärenlampi SO (2007) Production of transgenic strawberries by temporary immersion bioreactor system and verification by TAIL-PCR. BMC Biotechnol 7:11
Hobbs SL, Kpodar P, DeLong CM (1990) The effect of T-DNA copy number, position and methylation on reporter gene expression in tobacco transformants. Plant Mol Biol 15:851–864
Holster M, de Waele D, Depicker A, Messens E, Montagu VM (1978) Transformation and transfection of Agrobacterium tumefaciens. Mol Gen Genet 163:181–187
Hostetter MK (1994) Adhesins and ligands involved in the interaction of Candida spp. with epithelial and endothelial surfaces. Clin Microbiol Rev 7(1):29–42
Hussey RS, Grundler FMW (1998) Nematode parasitism of plants. In: Perry RN, Wright DT (eds) The physiology and biochemistry of free-living and plant-parasitic nematodes. CABI Publishing, Wallingford, pp 213–243
Jansson HB, Jeyaprakash A, Coles GC, Marban-Mendoza N, Zuckerman BM (1986) Fluorescent and ferritin labelling of cuticle surface carbohydrates of Caenorhabditis elegans and Panagrellus redivivus. J Nematol 18:570–574
Jones MGK (1981) The development and function of plant cells modified by endoparasitic nematodes. In: Zuckerman BM, Rohde RA (eds) Plant parasitic nematodes, vol 3. Academic Press, New York, pp 255–279
Kalariya HM (2010) Characterization of transgenic plum lines expressing gastrodia antifungal protein (gafp). Thesis submitted to University of Clemson University, Clemson
Kamble S, Misra HS, Mahajan SK, Eapen S (2003) A protocol for efficient biolistic transformation of moth bean Vigna aconitifolia L. Plant Mol Biol Rep 21:457a–457j
Kohli A, Gahakwa D, Vain P, Laurie DA, Christou P (1999) Transgene expression in rice engineered through particle bombardment: molecular factors controlling stable expression and transgene silencing. Planta 208:88–97
Konig A, Cockburn A, Crevel R, Debruyne E, Grafstroem R, Hammerling U (2004) Assessment of The safety of foods derived from genetically modified (GM) crops. Food Chem Toxicol 42:1047–1088
Liener IE, Hill EG (1953) The effect of heat treatment of the nutritive value and hemagglutinating activity of soybean oil meal. J Nutr 49(4):609–620
Lin HJ, McClure MA (1996) Surface coat of Meloidogyne incognita. J Nematol 28:216–224
Lis H, Sharon N (1998) Lectins: carbohydrate-specific proteins that mediate cellular recognition. Chem Rev 98(2):637–674
Liu YG, Mitsukawa N, Oosumi T, Whittier RF (1995) Efficient isolation and mapping of Arabidopsis thaliana T-DNA insert junctions by thermal asymmetric interlaced PCR. Plant J 8(3):457–463
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193(1):265–275
McClure MA, Stynes BA (1988) Lectin binding sites on the amphidial exudates of Meloidogyne. J Nematol 20:321–326
McClure MA, Zuckerman BM (1982) Localization of cuticular binding sites of Concanavalin A on Caenorhabditis elegans and Meloidogyne incognita. J Nematol 14:39–44
Milligan SB, Bodeau J, Yaghoobi J, Kaloshian I, Zabel P, Williamson VM (1998) The root knot nematode resistance gene Mi from tomato is a member of the leucine zipper, nucleotide binding, leucine-rich repeat family of plant genes. Plant Cell 10(8):1307
Moens M, Perry R, Starr J (2009) Meloidogyne species: a diverse group of novel and important plant parasites. In: Perry RN, Moens M, Starr JL (eds) Root-knot nematodes. Wallingford, UK, pp 483–490
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Nagel A (2010) Understanding GAFP, a plant lectin with broad spectrum inhibitory activity. Thesis submitted to Clemson University, Clemson
Nagel AK, Scorza R, Petri C, Schnabel G (2008) Generation and characterization of transgenic plum lines expressing the gastrodia-anti fungal protein. HortScience 43:1514–1521
Neekhra V (2009) Cloning lectin gene from Remusatia vivipara, and the nematicidal activity of lectin expressed in Escherichia coli. Thesis submitted to University of Agricultural Sciences, Dharwad, India
Neekhra V, Bhat GG, Bhagat YS, Lingaraju S, Bhat RS (2011) Nematicidal activity of Remusatia vivipara lectin expressed in Escherichia coli. Curr Sci 101(2):150–151
Ogwulumba SI, Ugwuoke KI (2013) Coeficient and path analyses of the impact of root gals caused by Meloidogyne javanica on some growth and yield parameters of tomato (Solanum lycopersicum). Int J Plant Soil Sci 2(2):22–29
Ohri P, Pannu SK (2010) Effect of phenolic compounds on nematodes: a review. J Appl Nat Sci 2(2):344–350
Peumans WJ, Van Damme EJM (1995) Lectins as plant defense proteins. Plant Physiol 109(2):347–352
Powell KS, Spence J, Brarathi M, Gatehouse JA, Gatehouse AMR (1998) Immunohistochemical and developmental studies to elucidate the mechanism of action of the snowdrop lectin on the rice brown planthopper, Nilaparvata lugens (Stal). J Insect Physiol 44:529–539
Ripoll C, Favery B, Lecomte P, Van Damme E, Peumans W, Abad P, Jouanin L (2003) Evaluation of the ability of lectin from snowdrop (Galanthus nivalis) to protect plants against root-knot nematodes. Plant Sci 164:517–523
Sadasivam S, Manickam A (2009) Biochemical Methods. New Age International (T) Ltd, Publishers, New Delhi, pp 147–148
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Sasser JN, Eisenback JD, Carter CC, Triantaphyllou AC (1983) The International Meloidogyne project—its goals and accomplishments. Annu Rev Phytopathol 21:271–288
Sijmons PC, Cardol EF, Goddijn OJM (1994) Gene activities in nematode-induced feeding structures. In: Daniels MJ, Downie MJ, Osbourn AE (eds) Advances in molecular genetics of plant-microbe interactions. Kluwer Academic Publishers, Netherland, pp 333–338
Singh S, Singh B, Singh AP (2015) Nematodes: a threat to sustainability of agriculture. Procedia Environ Sci 29:215–216
Swamy BM, Hegde GV, Naik RS, Inamdar SR (2001) T-antigen binding lectin from the phytopathogenic fungus Sclerotium rolfsii. Biol Biochem Clin Biochem 15:45–55
Taylor AL, Sasser JN (1978) Biology, identification and control of root-knot nematodes (Meloidogyne species). Cooperative Publications of the Department of Plant Pathology, North Carolina State University & U.S. Agency, International Development, Raleigh, pp 111
Trigueros V, Lougarre A, Ali-Ahmed D, Rahbe Y, Guillot J, Chavant L, Fournier D, Paquereau L (2003) Xerocomus chrysenteron lectin: identification of a new pesticidal protein. Biochim Biophys Acta 1621(3):292–298
Upadhyaya NM, Zhu QH, Zhou XR, Eamens AL, Hoque MS, Ramm K, Shivakkumar R, Smith KF, Pan ST, Li S, Peng K, Kim SJ, Dennis ES (2006) Dissociation (Ds) constructs, mapped Ds launch pads and a transiently-expressed transposase system suitable for localized insertional mutagenesis in rice. Theor Appl Genet 112(7):1326–1341
Urwin PE, Atkinson HJ, Waller DA, McPherson MJ (1995) Engineered oryzacystatin-I expressed in transgenic hairy roots confers resistance to Globodera pallida. Plant J 8:121–131
Urwin PE, Lilley CJ, McPherson MJ, Atkinson HJ (1997) Resistance to both cyst and root-knot nematodes conferred by transgenic Arabidopsis expressing a modified plant cystatin. Plant J 12:455–461
Van Damme EJM, Peumans WJ, Barre A, Rouge P (1998) Plant lectins: a composite of several distinct families of structurally and evolutionary related proteins with diverse biological roles. Crit Rev Plant Sci 17(6):575–692
Varrot A, Basheer SM, Imberty A (2013) Fungal lectins: structure, function and potential applications. Curr Opin Struct Biol 23:1–8
Williamson VM, Hussey RS (1996) Nematode pathogenesis and resistance in plants. Plant Cell 8:1735–1745
Yatohgo T, Nakata M, Tsumuraya Y, Hashimoto Y, Yamamoto S (1988) Purification and properties of a lectin from the fruit bodies of Flammulina velutipes. Agric Biol Chem 52(6):1485–1493
Zhao S, Guo YX, Liu QH, Wang HX, Ng TB (2009) Lectins but not antifungal proteins exhibit anti-nematode activity. Environ Toxicol Pharmacol 28(2):265–280
Zuckerman BM (1983) Hypotheses and possibilities of intervention in nematode chemoresponses. J Nematol 15:173–182
Acknowledgements
The authors thank Dr. Ramesh Aggarwal (CCMB, Hyderabad, India) and Dr. Thumballi Ganapathi (BARC, Mumbai, India) for useful discussions and for providing facility to carry out southern hybridization. We thank DBT, New Delhi (India) and UAS, Dharwad (India) for financial support to carry out this research work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bhagat, Y.S., Bhat, R.S., Kolekar, R.M. et al. Remusatia vivipara lectin and Sclerotium rolfsii lectin interfere with the development and gall formation activity of Meloidogyne incognita in transgenic tomato. Transgenic Res 28, 299–315 (2019). https://doi.org/10.1007/s11248-019-00121-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-019-00121-w