Abstract
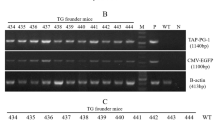
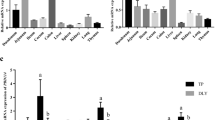
Use of huge amounts of antibiotics in farm animal production has promoted the prevalence of antibiotic-resistant bacteria, which poses a serious threat to public health. Therefore, alternative approaches are needed to reduce or replace antibiotic usage in the food animal industry. PR-39 is a pig-derived proline-rich antimicrobial peptide that has a broad spectrum of antibacterial activity and a low propensity for development of resistance by microorganisms. To test whether ubiquitous expression of PR-39 in transgenic (TG) mice can increase resistance against bacterial infection, we generated TG mice that ubiquitously express a pig-derived antimicrobial peptide PR-39 and analyzed their growth and resistance to infection of the highly pathogenic Actinobacillus pleuropneumoniae (APP) isolated from swine. The growth performance was significantly increased in TG mice compared with their wild-type (WT) littermates. After the APP challenge, TG mice exhibited a significantly higher survival rate and significantly lower tissue bacterial load than WT littermates. Furthermore, the tissue lesion severity that resulted from APP infection was milder in TG mice than that in their WT littermates. This study provides a good foundation for the development of PR-39-expressing TG animals, which could reduce the use of antibiotics in the farm animal industry.








Similar content being viewed by others
References
Aarestrup FM (2005) Veterinary drug usage and antimicrobial resistance in bacteria of animal origin. Basic Clin Pharmacol Toxicol 96:271–281. https://doi.org/10.1111/j.1742-7843.2005.pto960401.x
Ageitos JM, Sanchez-Perez A, Calo-Mata P, Villa TG (2016) Antimicrobial peptides (AMPs): ancient compounds that represent novel weapons in the fight against bacteria. Biochem Pharmacol. https://doi.org/10.1016/j.bcp.2016.09.018
Agerberth B, Lee JY, Bergman T, Carlquist M, Boman HG, Mutt V, Jornvall H (1991) Amino acid sequence of PR-39. Isolation from pig intestine of a new member of the family of proline-arginine-rich antibacterial peptides. Eur J Biochem 202:849–854
Auger E, Deslandes V, Ramjeet M, Contreras I, Nash JH, Harel J, Gottschalk M, Olivier M, Jacques M (2009) Host-pathogen interactions of Actinobacillus pleuropneumoniae with porcine lung and tracheal epithelial cells. Infect Immun 77:1426–1441. https://doi.org/10.1128/IAI.00297-08
Chantziaras I, Boyen F, Callens B, Dewulf J (2014) Correlation between veterinary antimicrobial use and antimicrobial resistance in food-producing animals: a report on seven countries. J Antimicrob Chemother 69:827–834. https://doi.org/10.1093/jac/dkt443
Cheng C, Sun WK, Liu R, Wang RM, Chen YH, Wang Y, Li JL, Lu XB, Gao R (2015) Comparison of gene expression of Toll-like receptors and antimicrobial peptides in immune organs and tissues between Yorkshire and Tibetan pigs. Anim Genet 46:272–279. https://doi.org/10.1111/age.12286
Cheung QC, Turner PV, Song C, Wu D, Cai HY, MacInnes JI, Li J (2008) Enhanced resistance to bacterial infection in protegrin-1 transgenic mice. Antimicrob Agents Chemother 52:1812–1819. https://doi.org/10.1128/AAC.01530-07
Fan F, Wu Y, Liu J (2010) Expression and purification of two different antimicrobial peptides, PR-39 and Protegrin-1 in Escherichia coli. Protein Expr Purif 73:147–151. https://doi.org/10.1016/j.pep.2010.05.012
Feng Y, Zhang H, Wu Z, Wang S, Cao M, Hu D, Wang C (2014) Streptococcus suis infection: an emerging/reemerging challenge of bacterial infectious diseases? Virulence 5:477–497. https://doi.org/10.4161/viru.28595
Gudmundsson GH, Magnusson KP, Chowdhary BP, Johansson M, Andersson L, Boman HG (1995) Structure of the gene for porcine peptide antibiotic PR-39, a cathelin gene family member: comparative mapping of the locus for the human peptide antibiotic FALL-39. Proc Natl Acad Sci USA 92:7085–7089
Hancock RE, Sahl HG (2006) Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol 24:1551–1557. https://doi.org/10.1038/nbt1267
Hardstaff JL, Marion G, Hutchings MR, White PC (2014) Evaluating the tuberculosis hazard posed to cattle from wildlife across Europe. Res Vet Sci 97(Suppl):S86–S93. https://doi.org/10.1016/j.rvsc.2013.12.002
Joshi M, Keith PH, Haisch C, Verbanac K (2008) Real-time PCR to determine transgene copy number and to quantitate the biolocalization of adoptively transferred cells from EGFP-transgenic mice. Biotechniques 45:247–258. https://doi.org/10.2144/000112913
Lee PH, Ohtake T, Zaiou M, Murakami M, Rudisill JA, Lin KH, Gallo RL (2005) Expression of an additional cathelicidin antimicrobial peptide protects against bacterial skin infection. Proc Natl Acad Sci USA 102:3750–3755. https://doi.org/10.1073/pnas.0500268102
Li L, Chen Z, Bei W, Su Z, Huang Q, Zhang L, Chen H, Zhou R (2015) Catecholamines promote Actinobacillus pleuropneumoniae growth by regulating iron metabolism. PLoS ONE 10:e121887. https://doi.org/10.1371/journal.pone.0121887
Linde CM, Hoffner SE, Refai E, Andersson M (2001) In vitro activity of PR-39, a proline-arginine-rich peptide, against susceptible and multi-drug-resistant Mycobacterium tuberculosis. J Antimicrob Chemother 47:575–580
Ramanathan B, Davis EG, Ross CR, Blecha F (2002) Cathelicidins: microbicidal activity, mechanisms of action, and roles in innate immunity. Microbes Infect 4:361–372
Salzman NH, Ghosh D, Huttner KM, Paterson Y, Bevins CL (2003) Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature 422:522–526. https://doi.org/10.1038/nature01520
Sang Y, Blecha F (2009) Porcine host defense peptides: expanding repertoire and functions. Dev Comp Immunol 33:334–343. https://doi.org/10.1016/j.dci.2008.05.006
Shi J, Ross CR, Chengappa MM, Blecha F (1994) Identification of a proline-arginine-rich antibacterial peptide from neutrophils that is analogous to PR-39, an antibacterial peptide from the small intestine. J Leukoc Biol 56:807–811
Storici P, Zanetti M (1993) A cDNA derived from pig bone marrow cells predicts a sequence identical to the intestinal antibacterial peptide PR-39. Biochem Biophys Res Commun 196:1058–1065. https://doi.org/10.1006/bbrc.1993.2358
Van Boeckel TP, Brower C, Gilbert M, Grenfell BT, Levin SA, Robinson TP, Teillant A, Laxminarayan R (2015) Global trends in antimicrobial use in food animals. Proc Natl Acad Sci USA 112:5649–5654. https://doi.org/10.1073/pnas.1503141112
Veldhuizen EJ, Schneider VA, Agustiandari H, van Dijk A, Tjeerdsma-van BJ, Bikker FJ, Haagsman HP (2014) Antimicrobial and immunomodulatory activities of PR-39 derived peptides. PLoS ONE 9:e95939. https://doi.org/10.1371/journal.pone.0095939
Vieira AR, Collignon P, Aarestrup FM, McEwen SA, Hendriksen RS, Hald T, Wegener HC (2011) Association between antimicrobial resistance in Escherichia coli isolates from food animals and blood stream isolates from humans in Europe: an ecological study. Foodborne Pathog Dis 8:1295–1301. https://doi.org/10.1089/fpd.2011.0950
Wang S, Zeng X, Yang Q, Qiao S (2016a) Antimicrobial peptides as potential alternatives to antibiotics in food animal industry. Int J Mol Sci. https://doi.org/10.3390/ijms17050603
Wang S, Zeng X, Yang Q, Qiao S (2016b) Antimicrobial peptides as potential alternatives to antibiotics in food animal industry. Int J Mol Sci. https://doi.org/10.3390/ijms17050603
Xiao H, Shao F, Wu M, Ren W, Xiong X, Tan B, Yin Y (2015) The application of antimicrobial peptides as growth and health promoters for swine. J Anim Sci Biotechnol 6:19. https://doi.org/10.1186/s40104-015-0018-z
Yang X, Cheng YT, Tan MF, Zhang HW, Liu WQ, Zou G, Zhang LS, Zhang CY, Deng SM, Yu L, Hu XY, Li L, Zhou R (2015) Overexpression of Porcine Beta-Defensin 2 Enhances Resistance to Actinobacillus pleuropneumoniae infection in pigs. Infect Immun 83:2836–2843. https://doi.org/10.1128/IAI.03101-14
Acknowledgements
This study was supported by a grant from the National Natural Science Foundation of China (grant no. 31601911) and two grants from the Department of Science and Technology of Guangdong Province, China (grant nos. 2014A030310500 and 2015TX01N081).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zeng, F., Dong, R., Zhao, C. et al. Constitutive expression of antimicrobial peptide PR-39 in transgenic mice significantly enhances resistance to bacterial infection and promotes growth. Transgenic Res 27, 409–422 (2018). https://doi.org/10.1007/s11248-018-0084-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-018-0084-z