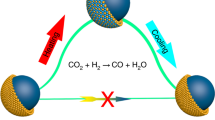
Abstract
Gold is examined here as an alternative to copper for the selective dehydrogenation of ethanol to acetaldehyde and hydrogen. Despite its high selectivity, gold is only active at temperatures higher than 250 °C for this reaction. We demonstrate that addition of a small amount of Ni on either supported or unsupported Au surfaces induces resistance to sintering, along with a beneficial effect on the catalytic activity. NiAu alloys prepared here with Ni as the minority component to the limit of atomic dispersion in the gold surfaces, catalyze the reaction beginning below 150 °C. A significant decrease of the apparent activation energy from 96 ± 3 kJ/mol for the monometallic Au to 59 ± 5 kJ/mol for the alloy was found. The Ni dispersion and concentration as a function of gas environment was followed by in situ DRIFTS and by XPS. The stability of the catalyst morphology was investigated through post-reaction microscopy imaging and long-term stability tests under reaction conditions. As shown via dynamic reaction experiments, acetaldehyde and H2 were selectively produced up to 280 °C. A small drop of selectivity at higher temperatures is attributed to the formation of Ni clusters, as proven by CO-DRIFTS on the used sample. Comparison with samples of higher Ni loading, where Ni clusters are formed, clearly shows that they catalyze the undesired full decomposition of ethanol to CO, CH4, and H2.











Similar content being viewed by others
References
Sun J, Karim AM, Mei D et al (2015) New insights into reaction mechanisms of ethanol steam reforming on Co-ZrO2. Appl Catal B Environ 162:141–148. https://doi.org/10.1016/j.apcatb.2014.06.043
Sanchez-Sanchez MC, Navarro Yerga RM, Kondarides DI et al (2010) Mechanistic aspects of the ethanol steam reforming reaction for hydrogen production on Pt, Ni, and PtNi catalysts supported on gamma-Al2O3. J Phys Chem A 114:3873–3882. https://doi.org/10.1021/jp906531x
Eckert M, Fleischmann G, Jira R et al (2012) Acetaldehyde. In: Ullmann’s Encycl. Ind. Chem, Wiley, Weinheim, pp 191–207
Shan J, Janvelyan N, Li H et al (2017) Selective non-oxidative dehydrogenation of ethanol to acetaldehyde and hydrogen on highly dilute NiCu alloys. Appl Catal B Environ 205:541–550. https://doi.org/10.1016/j.apcatb.2016.12.045
Tu YJ, Chen YW, Li C (1994) Characterization of unsupported copper-chromium catalysts for ethanol dehydrogenation. J Mol Catal 89:179–189. https://doi.org/10.1016/0304-5102(93)E0331-A
Santacesaria E, Carotenuto G, Tesser R, Di Serio M (2012) Ethanol dehydrogenation to ethyl acetate by using copper and copper chromite catalysts. Chem Eng J 179:209–220. https://doi.org/10.1016/j.cej.2011.10.043
Tu Y-J, Chen Y-W (2001) Effects of alkali metal oxide additives on Cu/SiO 2 catalyst in the dehydrogenation of ethanol. Ind Eng Chem Res 40:5889–5893. https://doi.org/10.1021/ie010272q
Mavrikakis M, Stoltze P, Nørskov JK (2000) Making gold less noble. Catal Lett 64:101–106
Hammer B, Norskov JK (1995) Why gold is the noblest of all metals. Nature 376:238–240
Zugic B, Wang L, Heine C et al (2016) Dynamic restructuring drives catalytic activity on nanoporous gold–silver alloy catalysts. Nat Mater. https://doi.org/10.1038/NMAT4824
Schimpf S, Lucas M, Mohr C et al (2002) Supported gold nanoparticles: in-depth catalyst characterization and application in hydrogenation and oxidation reactions. Catal Today. https://doi.org/10.1016/S0920-5861(01)00479-5
Claus P (2005) Heterogeneously catalysed hydrogenation using gold catalysts. Appl Catal A Gen. https://doi.org/10.1016/j.apcata.2004.12.048
Tyo EC, Yin CR, Di Vece M et al (2012) Oxidative dehydrogenation of cyclohexane on cobalt oxide (Co3O4) nanoparticles: the effect of particle size on activity and selectivity. ACS Catal 2:2409–2423. https://doi.org/10.1021/cs300479a
Hutchings GJ (2002) Gold catalysis in chemical processing. Catal Today 72:11–17. https://doi.org/10.1016/S0920-5861(01)00473-4
Fu Q, Saltsburg H, Flytzani-Stephanopoulos M (2003) Active nonmetallic Au and Pt species on ceria-based water-gas shift catalysts. Science 301:935–938. https://doi.org/10.1126/science.1085721
Fu Q, Deng W, Saltsburg H, Flytzani-Stephanopoulos M (2005) Activity and stability of low-content gold-cerium oxide catalysts for the water-gas shift reaction. Appl Catal B Environ. https://doi.org/10.1016/j.apcatb.2004.07.015
Deng W, Frenkel AI, Si R, Stephanopoulos MF (2008) Reaction-relevant Au structures in the low temperature WGS reaction on Au–CeO2. J Phys Chem C 112:12834–12840. https://doi.org/10.1021/jp800075y
Yi N, Si R, Saltsburg H, Flytzani-Stephanopoulos M (2010) Steam reforming of methanol over ceria and gold-ceria nanoshapes. Appl Catal B Environ. https://doi.org/10.1016/j.apcatb.2009.12.012
Yi N, Si R, Saltsburg H, Flytzani-Stephanopoulos M (2010) Active gold species on cerium oxide nanoshapes for methanol steam reforming and the water gas shift reactions. Energy Environ Sci 3:831. https://doi.org/10.1039/b924051a
Boucher MB, Goergen S, Yi N, Flytzani-Stephanopoulos M (2011) “Shape effects” in metal oxide supported nanoscale gold catalysts. Phys Chem Chem Phys 13:2517–2527. https://doi.org/10.1039/c0cp02009e
Wang C, Boucher M, Yang M et al (2014) ZnO-modified zirconia as gold catalyst support for the low-temperature methanol steam reforming reaction. Appl Catal B Environ 154–155:142–152. https://doi.org/10.1016/j.apcatb.2014.02.008
Garbarino G, Wang C, Valsamakis I et al (2017) Acido-basicity of lanthana/alumina catalysts and their activity in ethanol conversion. Appl Catal B Environ 200:458–468. https://doi.org/10.1016/j.apcatb.2016.07.010
Haruta M (2007) New generation of gold catalysts: nanoporous foams and tubes—is unsupported gold catalytically active?. ChemPhysChem 8:1911–1913. https://doi.org/10.1002/cphc.200700325
Wittstock A, Zielasek V, Biener J et al (2010) Nanoporous gold catalysts for selective gas-phase oxidative coupling of methanol at low temperature. Science 327:319–322. https://doi.org/10.1126/science.1183591
Juarez T, Biener J, Weissmüller J, Hodge AM (2017) Nanoporous metals with structural hierarchy: a review. Adv Eng Mater 1700389:1–23. https://doi.org/10.1002/adem.201700389
Besenbacher F, Chorkendorff I, Clausen BS et al (1998) Design of a surface alloy catalyst for steam reforming. Science 279:1913–1915
Suzuki K, Yamaguchi T, Matsushita K et al (2013) Aerobic oxidative esterification of aldehydes with alcohols by gold-nickel oxide nanoparticle catalysts with a core-shell structure. ACS Catal 3:1845–1849. https://doi.org/10.1021/cs4004084
Masoud N, Delannoy L, Calers C et al (2017) Silica-supported Au–Ag catalysts for the selective hydrogenation of butadiene. ChemCatChem 9:2418–2425. https://doi.org/10.1002/cctc.201700127
Xu B, Siler CGF, Madix RJ, Friend CM (2014) Ag/Au mixed sites promote oxidative coupling of methanol on the alloy surface. Chem Eur J 20:4646–4652. https://doi.org/10.1002/chem.201304837
Delannoy L, Thrimurthulu G, Reddy PS et al (2014) Selective hydrogenation of butadiene over TiO2 supported copper, gold and gold-copper catalysts prepared by deposition-precipitation. Phys Chem Chem Phys 16:26514–26527. https://doi.org/10.1039/c4cp02141j
Sandoval A, Louis C, Zanella R (2013) Improved activity and stability in CO oxidation of bimetallic Au-Cu/TiO2 catalysts prepared by deposition-precipitation with urea. Appl Catal B Environ 140–141:363–377. https://doi.org/10.1016/j.apcatb.2013.04.039
Lim JE, Lee UJ, Ahn SH et al (2015) Oxygen reduction reaction on electrodeposited PtAu alloy catalysts in the presence of phosphoric acid. Appl Catal B Environ 165:495–502. https://doi.org/10.1016/j.apcatb.2014.10.042
El Kolli N, Delannoy L, Louis C (2013) Bimetallic Au-Pd catalysts for selective hydrogenation of butadiene: Influence of the preparation method on catalytic properties. J Catal. https://doi.org/10.1016/j.jcat.2012.09.022
Griffin MB, Rodriguez AA, Montemore MM et al (2013) The selective oxidation of ethylene glycol and 1,2-propanediol on Au, Pd, and Au-Pd bimetallic catalysts. J Catal 307:111–120. https://doi.org/10.1016/j.jcat.2013.07.012
Carter JH, Althahban S, Nowicka E et al (2016) Synergy and anti-synergy between palladium and gold in nanoparticles dispersed on a reducible support. ACS Catal. https://doi.org/10.1021/acscatal.6b01275
Zhang Y, Diao W, Williams CT, Monnier JR (2014) Selective hydrogenation of acetylene in excess ethylene using Ag- and Au-Pd/SiO2 bimetallic catalysts prepared by electroless deposition. Appl Catal A Gen. https://doi.org/10.1016/j.apcata.2013.10.024
Guan Y, Hensen EJM (2013) Selective oxidation of ethanol to acetaldehyde by Au-Ir catalysts. J Catal 305:135–145. https://doi.org/10.1016/j.jcat.2013.04.023
Kyriakou G, Boucher MB, Jewell AD et al (2012) Isolated metal atom geometries as a strategy for selective heterogeneous hydrogenations. Science 335:1209–1212. https://doi.org/10.1126/science.1215864
Aich P, Wei H, Basan B et al (2015) Single-atom alloy Pd-Ag catalyst for selective hydrogenation of acrolein. J Phys Chem C. https://doi.org/10.1021/acs.jpcc.5b01357
Boucher MB, Marcinkowski MD, Liriano ML et al (2013) Molecular-scale perspective of water-catalyzed methanol dehydrogenation to formaldehyde. ACS Nano 7:6181–6187. https://doi.org/10.1021/nn402055k
Shan J, Lucci FR, Liu J et al (2016) Water co-catalyzed selective dehydrogenation of methanol to formaldehyde and hydrogen. Surf Sci 650:121–129. https://doi.org/10.1016/j.susc.2016.02.010
Lucci FR, Liu J, Marcinkowski MD et al (2015) Selective hydrogenation of 1,3-butadiene on platinum–copper alloys at the single-atom limit. Nat Commun doi. https://doi.org/10.1038/ncomms9550
Pei GX, Liu XY, Wang A et al (2015) Ag alloyed Pd single-atom catalysts for efficient selective hydrogenation of acetylene to ethylene in excess ethylene. ACS Catal 5:3717–3725. https://doi.org/10.1021/acscatal.5b00700
Pei GX, Liu XY, Yang X et al (2017) Performance of Cu-alloyed Pd single-atom catalyst for semihydrogenation of acetylene under simulated front-end conditions. ACS Catal 1491–1500. https://doi.org/10.1021/acscatal.6b03293
Boucher MB, Zugic B, Cladaras G et al (2013) Single atom alloy surface analogs in Pd0.18Cu15 nanoparticles for selective hydrogenation reactions. Phys Chem Chem Phys 15:12187–12196. https://doi.org/10.1039/c3cp51538a
Liu J, Lucci FR, Yang M et al (2016) Tackling CO poisoning with single-atom alloy catalysts. J Am Chem Soc 138:6396–6399. https://doi.org/10.1021/jacs.6b03339
Liu J, Shan J, Lucci FR et al (2017) Palladium-gold single atom alloy catalysts for liquid phase selective hydrogenation of 1-hexyne. Catal Sci Technol. https://doi.org/10.1039/C7CY00794A
Lucci FR, Darby MT, Mattera MFG et al (2016) Controlling hydrogen activation, spillover, and desorption with Pd-Au single-atom alloys. J Phys Chem Lett 7:480–485. https://doi.org/10.1021/acs.jpclett.5b02400
Wang Z-T, Darby MT, Therrien AJ et al (2016) Preparation, structure, and surface chemistry of Ni–Au single atom alloys. J Phys Chem C 120:13574–13580. https://doi.org/10.1021/acs.jpcc.6b03473
Guan Y, Hensen EJM (2009) Ethanol dehydrogenation by gold catalysts: the effect of the gold particle size and the presence of oxygen. Appl Catal A Gen 361:49–56. https://doi.org/10.1016/j.apcata.2009.03.033
Jiang K, Siahrostami S, Li Y, Lu Z, Gardener J, Lattimer J, Stokes C, Hill W, Bell D, Chan K, Nørskov JK, Yi Cui Y, Wang H (2017) Transition metal atoms in a graphene shell as active centers for highly efficient artificial photosynthesis. Nat Energy 1–11
Ruban A, Hammer B, Stoltze P et al (1997) Surface electronic structure and reactivity of transition and noble metals. J Mol Catal A Chem. https://doi.org/10.1016/S1381-1169(96)00348-2
De Souza G, Balzaretti NM, Marcílio NR, Perez-Lopez OW (2012) Decomposition of ethanol over Ni-Al catalysts: effect of copper addition. Procedia Eng 42:335–345. https://doi.org/10.1016/j.proeng.2012.07.425
Mattos LV, Jacobs G, Davis BH, Noronha FB (2012) Production of hydrogen from ethanol: review of reaction mechanism and catalyst deactivation. Chem Rev 112:4094–4123. https://doi.org/10.1021/cr2000114
Tedsree K, Li T, Jones S et al (2011) Hydrogen production from formic acid decomposition at room temperature using a Ag–Pd core–shell nanocatalyst. Nat Nanotechnol 6:302–307. https://doi.org/10.1038/nnano.2011.42
Elmasry MAA, Gaber A, Khater EMH (1998) Thermal decomposition of Ni(II) and Fe(III) nitrates and their mixture. J Therm Anal Calorim 52:489–495. https://doi.org/10.1023/A:1010155203247
Rodriguez JA, Hanson JC, Frenkel AI et al (2002) Experimental and theoretical studies on the reaction of H2 with NiO: role of O vacancies and mechanism for oxide reduction. J Am Chem Soc 124:346–354. https://doi.org/10.1021/ja0121080
Walton J, Wincott P, Fairley N, Carrick A (2010) Peak Fitting with CasaXPS. Acolyte Science, Knutsford
Chai M, Liu X, Li L et al (2014) SiO2-supported Au-Ni bimetallic catalyst for the selective hydrogenation of acetylene. Chin J Catal 38:1338–1346. https://doi.org/10.1016/S1872
Sun Y, Xia Y (2002) Shape-controlled synthesis of gold and silver nanoparticles. Science 298:2176–2179. https://doi.org/10.1126/science.1077229
Wang YQ, Liang WS, Geng CY (2009) Coalescence behavior of gold nanoparticles. Nanoscale Res Lett 4:684–688. https://doi.org/10.1007/s11671-009-9298-6
Tynkova A, Sidorenko S, Voloshko S et al (2013) Interdiffusion in Au(120 nm)/Ni(70 nm) thin films at the low-temperature annealing in the different atmospheres. Vacuum 87:69–74. https://doi.org/10.1016/j.vacuum.2012.07.005
Tenney SA, He W, Roberts CC et al (2011) CO-induced diffusion of Ni atoms to the surface of Ni-Au clusters on TiO2(110). J Phys Chem C. https://doi.org/10.1021/jp2014258
Skriver HL, Rosengaard NM (1992) Surface energy and work function of elemental metals. Phys Rev B 46:7157–7168. https://doi.org/10.1103/PhysRevB.46.7157
Prieto P, Nistor V, Nouneh K et al (2012) XPS study of silver, nickel and bimetallic silver-nickel nanoparticles prepared by seed-mediated growth. Appl Surf Sci 258:8807–8813. https://doi.org/10.1016/j.apsusc.2012.05.095
D’Addato S, Grillo V, Altieri S et al (2011) Structure and stability of nickel/nickel oxide core–shell nanoparticles. J Phys Condens Matter 23:175003. https://doi.org/10.1088/0953-8984/23/17/175003
Mihaylov M, Knözinger H, Hadjiivanov K, Gates BC (2007) Characterization of the oxidation states of supported gold species by IR spectroscopy of adsorbed CO. Chem Ing Tech 79:795–806. https://doi.org/10.1002/cite.200700029
Moya SF, Martins RL, Schmal M (2011) Monodispersed and nanostructrured Ni/SiO2 catalyst and its activity for non oxidative methane activation. Appl Catal A Gen 396:159–169. https://doi.org/10.1016/j.apcata.2011.02.007
Poncelet G, Centeno MA, Molina R (2005) Characterization of reduced α-alumina-supported nickel catalysts by spectroscopic and chemisorption measurements. Appl Catal A Gen 288:232–242. https://doi.org/10.1016/j.apcata.2005.04.052
Mihaylov M, Tsoncheva T, Hadjiivanov K (2011) Structure sensitivity of methanol decomposition on Ni/SiO2 catalysts. J Mater Sci 46:7144–7151. https://doi.org/10.1007/s10853-011-5437-4
Trant AG, Jones TE, Gustafson J et al (2009) Alloy formation in the Au{1 1 1}/Ni system—an investigation with scanning tunnelling microscopy and medium energy ion scattering. Surf Sci 603:571–579. https://doi.org/10.1016/j.susc.2008.12.028
Liu X, Xu B, Haubrich J et al (2009) Surface-mediated self-coupling of ethanol on gold. J Am Chem Soc 131:5757–5759. https://doi.org/10.1021/ja900822r
Jørgensen B, Egholm Christiansen S, Dahl Thomsen ML, Christensen CH (2007) Aerobic oxidation of aqueous ethanol using heterogeneous gold catalysts: efficient routes to acetic acid and ethyl acetate. J Catal 251:332–337. https://doi.org/10.1016/j.jcat.2007.08.004
Madix RJ, Friend CM, Liu XY (2008) Anticipating catalytic oxidation reactions on gold at high pressure (including liquid phase) from ultrahigh vacuum studies. J Catal 258:410–413. https://doi.org/10.1016/j.jcat.2008.06.026
Acknowledgements
This material is based upon work supported as part of the Integrated Mesoscale Architectures for Sustainable Catalysis (IMASC), an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Award Number DE- SC0012573. Work at LLNL was performed under the auspices of the U.S. Department of Energy by LLNL under Contract DE-AC52-07NA27344. TEM and SEM imaging was performed at Harvard University’s Center for Nanoscale Systems (CNS), a member of the National Nanotechnology Infrastructure Network (NNIN), which is supported by the National Science Foundation under NSF Award No. 1541959.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Giannakakis, G., Trimpalis, A., Shan, J. et al. NiAu Single Atom Alloys for the Non-oxidative Dehydrogenation of Ethanol to Acetaldehyde and Hydrogen. Top Catal 61, 475–486 (2018). https://doi.org/10.1007/s11244-017-0883-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-017-0883-0