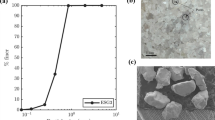
Abstract
No matter how sophisticated the structures are and on what length scale the pore sizes are, fluid displacement in porous media can be visualized, captured, mimicked and optimized using microfluidics. Visualizing transport processes is fundamental to our understanding of complex hydrogeological systems, petroleum production, medical science applications and other engineering applications. Microfluidics is an ideal tool for visual observation of flow at high temporal and spatial resolution. Experiments are typically fast, as sample volume is substantially low with the use of miniaturized devices. This review first discusses the fabrication techniques for generating microfluidics devices, experimental setups and new advances in microfluidic fabrication using three-dimensional printing, geomaterials and biomaterials. We then address multiphase transport in subsurface porous media, with an emphasis on hydrology and petroleum engineering applications in the past few decades. We also cover the application of microfluidics to study membrane systems in biomedical science and particle sorting. Lastly, we explore how synergies across different disciplines can lead to innovations in this field. A number of problems that have been resolved, topics that are under investigation and cutting-edge applications that are emerging are highlighted.









Similar content being viewed by others
References
Adrian, R.J., Westerweel, J.: Particle Image Velocimetry. Cambridge University Press, Cambridge (2011)
Ahmed, F.E., Lalia, B.S., Hashaikeh, R.: A review on electrospinning for membrane fabrication: challenges and applications. Desalination 356, 15–30 (2015)
Alagorni, A.H., Yaacob, Z., Nour, A.H.: An overview of oil production stages: enhanced oil recovery techniques and nitrogen injection. Int. J. Environ. Sci. Dev. 6(9), 693–701 (2015)
Albani, J.R.: Structure and Dynamics of Macromolecules: Absorption and Fluorescence Studies. Elsevier, Amsterdam (2011)
Albaugh, K.B.: Electrode phenomena during anodic bonding of silicon to sodium borosilicate glass. J. Electrochem. Soc. 138(10), 3089–3094 (1991)
Alzahid, Y., et al.: Alkaline surfactant polymer flooding: what happens at the pore scale? In: SPE Europec Featured at 79th EAGE Conference and Exhibition. Society of Petroleum Engineers (2017)
Alzahid, Y.A., et al.: Functionalisation of polydimethylsiloxane (PDMS)—microfluidic devices coated with rock minerals. Sci. Rep. 8(1), 15518 (2018)
Alzahid, Y.A., Mostaghimi, P., Walsh, S.D.C., Armstrong, R.T.: Flow regimes during surfactant flooding: the influence of phase behaviour. Fuel 236, 851–860 (2019)
Amirian, T., Haghighi, M., Mostaghimi, P.: Pore scale visualization of low salinity water flooding as an enhanced oil recovery method. Energy Fuels 31, 13133–13143 (2017)
Anbari, A., et al.: Microfluidic model porous media: fabrication and applications. Small 14(18), 1703575 (2018)
Armstrong, R.T., Berg, S.: Interfacial velocities and capillary pressure gradients during Haines jumps. Phys. Rev. E 88(4), 043010 (2013)
Atencia, J., Beebe, D.: Controlled microfluidic interfaces. Nature 437, 648–655 (2005)
Bachu, S.: Sequestration of CO2 in geological media: criteria and approach for site selection in response to climate change. Energy Convers. Manag. 41(9), 953–970 (2000)
Barisam, M., Saidi, M., Kashaninejad, N., Vadivelu, R., Nguyen, N.-T.: Numerical simulation of the behavior of toroidal and spheroidal multicellular aggregates in microfluidic devices with microwell and U-shaped barrier. Micromachines 8(12), 358 (2017)
Bartels, W.-B., et al.: Oil configuration under high-salinity and low-salinity conditions at pore scale: a parametric investigation by use of a single-channel micromodel. SPE J. 22(05), 1362–1373 (2017)
Bartels, W.B., et al.: Low salinity flooding (LSF) in sandstones at pore scale: micro-model development and investigation. In: SPE Annual Technical Conference and Exhibition. Society of Petroleum Engineers, p. 17, Dubai (2016)
Basabe-Desmonts, L., et al.: A simple approach to sensor discovery and fabrication on self-assembled monolayers on glass. J. Am. Chem. Soc. 126(23), 7293–7299 (2004)
Berkowski, K.L., Plunkett, K.N., Yu, Q., Moore, J.S.: Introduction to photolithography: preparation of microscale polymer silhouettes. J. Chem. Educ. 82(9), 1365 (2005)
Bhatia, S.N., Ingber, D.E.: Microfluidic organs-on-chips. Nat. Biotechnol. 32(8), 760–772 (2014)
Biswas, R., Lewis, J.E., Maroncelli, M.: Electronic spectral shifts, reorganization energies, and local density augmentation of Coumarin 153 in supercritical solvents. Chem. Phys. Lett. 310, 485–494 (1999)
Booth, R., Kim, H.: Characterization of a microfluidic in vitro model of the blood–brain barrier (μBBB). Lab Chip 12(10), 1784–1792 (2012)
Bowden, S.A., Tanino, Y., Akamairo, B., Christensen, M.: Recreating mineralogical petrographic heterogeneity within microfluidic chips: assembly, examples, and applications. Lab Chip 16(24), 4677–4681 (2016)
Brian, J.K., Ellis, M.: Review of polymer MEMS micromachining. J. Micromech. Microeng. 26(1), 013001 (2016)
Britt, L.K., Schoeffler, J.: The Geomechanics of a Shale Play: What Makes a Shale Prospective. Society of Petroleum Engineers, New York (2009)
Buchgraber, M., Kovscek, A.R., Castanier, L.M.: A study of microscale gas trapping using etched silicon micromodels. Transp. Porous Media 95(3), 647–668 (2012)
Cao, S.C., Dai, S., Jung, J.: Supercritical CO2 and brine displacement in geological carbon sequestration: micromodel and pore network simulation studies. Int. J. Greenhouse Gas Control 44(6), 104–114 (2016)
Chang, C., et al.: Pore-scale supercritical CO2 dissolution and mass transfer under imbibition conditions. Adv. Water Resour. 92(March), 142–158 (2016)
Chang, C., Zhou, Q., Oostrom, M., Kneafsey, T.J., Mehta, H.: Pore-scale supercritical CO2 dissolution and mass transfer under drainage conditions. Adv. Water Resour. 100, 14–25 (2017)
Chapman, E.M., Yang, J., Crawshaw, J.P., Boek, E.S.: Pore scale models for imbibition of CO2 analogue fluids in etched micro-model junctions using micro-fluidic experiments and direct flow calculations. Energy Proc. 37, 3680–3686 (2013)
Chen, Y.: Nanofabrication by electron beam lithography and its applications: a review. Microelectron. Eng. 135, 57–72 (2015)
Chen, Y., Li, Y., Valocchi, A.J., Christensen, K.T.: Lattice Boltzmann simulations of liquid CO2 displacing water in a 2D heterogeneous micromodel at reservoir pressure conditions. J. Contam. Hydrol. 53, 6178–6196 (2017)
Chrimes, A.F., Khoshmanesh, K., Stoddart, P.R., Mitchell, A., Kalantar-zadeh, K.: Microfluidics and Raman microscopy: current applications and future challenges. Chem. Soc. Rev. 42(13), 5880–5906 (2013)
Christensen, K.: The influence of peak-locking errors on turbulence statistics computed from PIV ensembles. Exp. Fluids 36(3), 484–497 (2004)
Datta, S., Chiang, H., Ramakrishnan, T.S., Weitz, D.: Spatial fluctuations of fluid velocities in flow through a three-dimensional porous medium. Phys. Rev. Lett. 111, 064501 (2013)
Datta, S.S., Dupin, J.-B., Weitz, D.A.: Fluid breakup during simultaneous two-phase flow through a three-dimensional porous medium. Phys. Fluids 26(6), 062004 (2014)
Davies, M.J., Marques, M.P.C., Radhakrishnan, A.N.P.: Chapter 2 Microfluidics Theory in Practice, Microfluidics in Detection Science: Lab-on-a-chip Technologies, pp. 29–60. The Royal Society of Chemistry, New York (2015)
de Haas, T.W., Fadaei, H., Guerrero, U., Sinton, D.: Steam-on-a-chip for oil recovery: the role of alkaline additives in steam assisted gravity drainage. Lab Chip 13(19), 3832–3839 (2013)
Deckert, V., et al.: Spatial resolution in Raman spectroscopy. Faraday Discuss. 177, 9–20 (2015)
Delamarche, E., Tonna, N., Lovchik, R.D., Bianco, F., Matteoli, M.: Pharmacology on microfluidics: multimodal analysis for studying cell–cell interaction. Curr. Opin. Pharmacol. 13(5), 821–828 (2013)
Dong, M., Liu, Q., Li, A.: Displacement mechanisms of enhanced heavy oil recovery by alkaline flooding in a micromodel. Particuology 10(3), 298–305 (2012)
Dong, Y., et al.: Microfluidics and circulating tumor cells. J. Mol. Diagn. 15(2), 149–157 (2013)
Doughty, M.J.: pH dependent spectral properties of sodium fluorescein ophthalmic solutions revisited. Ophthalmic Physiol. Opt. 30(2), 167–174 (2010)
Fakhari, A., Li, Y., Bolster, D., Christensen, K.T.: A phase-field lattice Boltzmann model for simulating multiphase flows in porous media: application and comparison to experiments of CO2 sequestration at pore scale. Adv. Water Resour. 114, 119–134 (2018)
Fan, X., et al.: A microfluidic chip integrated with a high-density PDMS-based microfiltration membrane for rapid isolation and detection of circulating tumor cells. Biosens. Bioelectron. 71, 380–386 (2015)
Franssila, S.: Introduction to Microfabrication. Wiley (2010)
Friend, J., Yeo, L.: Fabrication of microfluidic devices using polydimethylsiloxane. Biomicrofluidics 4(2), 026502 (2010)
Gerami, A., et al.: Microscale insights into gas recovery from bright and dull bands in coal. J. Petrol. Sci. Eng. 172, 373–382 (2018)
Gerami, A., et al.: Coal-on-a-chip: visualizing flow in coal fractures. Energy Fuels 31(10), 10393–10403 (2017)
Gerami, A., Mostaghimi, P., Armstrong, R.T., Zamani, A., Warkiani, M.E.: A microfluidic framework for studying relative permeability in coal. Int. J. Coal Geol. 159, 183–193 (2016)
Giboz, J., Copponnex, T., Mélé, P.: Microinjection molding of thermoplastic polymers: a review. J. Micromech. Microeng. 17(6), R96 (2007)
Godinez-Brizuela, O.E., Karadimitriou, N.K., Joekar-Niasar, V., Shore, C.A., Oostrom, M.: Role of corner interfacial area in uniqueness of capillary pressure-saturation- interfacial area relation under transient conditions. Adv. Water Resour. 107, 10–21 (2017)
Gomez, F.A.: The future of microfluidic point-of-care diagnostic devices. Bioanalysis 5(1), 1–3 (2013)
Gravesen, P., Branebjerg, J., Jensen, O.S.: Microfluidics—a review. J. Micromech. Microeng. 3(4), 168 (1993)
Guckenberger, D.J., de Groot, T.E., Wan, A.M.D., Beebe, D.J., Young, E.W.K.: Micromilling: a method for ultra-rapid prototyping of plastic microfluidic devices. Lab Chip 15(11), 2364–2378 (2015)
Gunda, N.S., Bera, B., Karadimitriou, N.K., Mitra, S.K., Hassanizadeh, S.M.: Reservoir-on-a-chip (ROC): a new paradigm in reservoir engineering. Lab Chip 11(22), 3785–3792 (2011)
Guo, H., et al.: Quantitative Raman spectroscopic investigation of geo-fluids high-pressure phase equilibria: part I. Accurate calibration and determination of CO2 solubility in water from 273.15 to 573.15 K and from 10 to 120 MPa. Fluid Phase Equilib. 382, 70–79 (2014)
Guo, H., Huang, Y., Chen, Y., Zhou, Q.: Quantitative Raman spectroscopic measurements of CO2 solubility in NaCl solution from (273.15 to 473.15) K at p = (10.0, 20.0, 30.0, and 40.0) MPa. J. Chem. Eng. Data 61(1), 466–474 (2015)
Haber, C.: Microfluidics in commercial applications; an industry perspective. Lab Chip 6(9), 1118–1121 (2006)
Hae Choi, Y., et al.: Effect of functional groups on the solubilities of coumarin derivatives in supercritical carbon dioxide. Chromatographia 47(1–2), 93–97 (1998)
Haeberle, S., Zengerle, R.: Microfluidic platforms for lab-on-a-chip applications. Lab Chip 7(9), 1094–1110 (2007)
Haszeldine, R.S.: Carbon capture and storage: how green can black be? Science (New York) 325(5948), 1647–1652 (2009)
He, K., Xu, L., Gao, Y., Yin, X., Neeves, K.B.: Evaluation of surfactant performance in fracturing fluids for enhanced well productivity in unconventional reservoirs using rock-on-a-Chip approach. J. Petrol. Sci. Eng. 135, 531–541 (2015)
He, K., Xu, L., Kenzhekhanov, S., Yin, X., Neeves, K.B.: A Rock-on-a-Chip Approach to Study Fluid Invasion and Flowback in Liquids-Rich Shale Formations. Society of Petroleum Engineers, London (2017)
Hematpour, H., Mardi, M., Edalatkhah, S., Arabjamaloei, R.: Experimental study of polymer flooding in low-viscosity oil using one-quarter five-spot glass micromodel. Pet. Sci. Technol. 29(11), 1163–1175 (2011)
Hilic, S., Boyer, S.V.A.E., AlAH, Pádua, Grolier, J.P.E.: Simultaneous measurement of the solubility of nitrogen and carbon dioxide in polystyrene and of the associated polymer swelling. J. Polym. Sci. Part B: Polym. Phys. 39(17), 2063–2070 (2001)
Homsy, G.M.: Viscous fingering in porous media. Annu. Rev. Fluid Mech. 19(1), 271–311 (1987)
Hu, R., Wan, J., Kim, Y., Tokunaga, T.K.: Wettability effects on supercritical CO2–brine immiscible displacement during drainage: pore-scale observation and 3D simulation. Int. J. Greenhouse Gas Control 60, 129–139 (2017a)
Hu, R., Wan, J., Kim, Y., Tokunaga, T.K.: Wettability Impact on Supercritical CO2 Capillary Trapping: Pore-Scale Visualization and Quantification. Water Resources Research, London (2017b)
Huh, D., Hamilton, G.A., Ingber, D.E.: From 3D cell culture to organs-on-chips. Trends Cell Biol. 21(12), 745–754 (2011)
Huh, D., et al.: Microfabrication of human organs-on-chips. Nat. Protoc. 8(11), 2135–2157 (2013)
Huh, D., et al.: Reconstituting organ-level lung functions on a chip. Science 328(5986), 1662–1668 (2010)
Huppert, H.E., Neufeld, J.A.: The fluid mechanics of carbon dioxide sequestration. Annu. Rev. Fluid Mech. 46(1), 255–272 (2014)
Iliescu, C., Taylor, H., Avram, M., Miao, J., Franssila, S.: A practical guide for the fabrication of microfluidic devices using glass and silicon. Biomicrofluidics 6(1), 016505–016505-16 (2012)
Jacob, R., Saylor, B.Z.: CO2 solubility in multi-component brines containing NaCl, KCl, CaCl 2 and MgCl 2 at 297 K and 1–14MPa. Chem. Geol. 424, 86–95 (2016)
Jafari, M., Jung, J.: Direct measurement of static and dynamic contact angles using a random micromodel considering geological CO2 sequestration. Sustainability 9(12), 2352 (2017)
Jahanshahi, A., Salvo, P., Vanfleteren, J.: PDMS selective bonding for the fabrication of biocompatible all polymer NC microvalves. J. Microelectromech. Syst. 22(6), 1354–1360 (2013)
Jiang, C., Tsukruk, V.V.: Freestanding nanostructures via layer-by-layer assembly. Adv. Mater. 18(7), 829–840 (2006)
Kalkandjiev, K., Gutzweiler, L., Welsche, M., Zengerle, R., Koltay, P.: A novel approach for the fabrication of all-polymer microfluidic devices. In: 2010 IEEE 23rd International Conference on Micro Electro Mechanical Systems (MEMS), pp. 1079–1082. IEEE (2010)
Kang, Y.-T., Doh, I., Byun, J., Chang, H.J., Cho, Y.-H.: Label-free rapid viable enrichment of circulating tumor cell by photosensitive polymer-based microfilter device. Theranostics 7(13), 3179 (2017)
Karadimitriou, N.K., Hassanizadeh, S.M.: A review of micromodels and their use in two-phase flow studies. Vadose Zone J. 11(3), 85 (2012)
Karadimitriou, N.K., Hassanizadeh, S.M., Joekar-Niasar, V., Kleingeld, P.J.: Micromodel study of two-phase flow under transient conditions: quantifying effects of specific interfacial area. Water Resour. Res. 50(10), 8125–8140 (2014)
Karadimitriou, N.K., Joekar-Niasar, V., Hassanizadeh, S.M., Kleingeld, P.J., Pyrak-Nolte, L.J.: A novel deep reactive ion etched (DRIE) glass micro-model for two-phase flow experiments. Lab Chip 12(18), 3413–3418 (2012)
Karadimitriou, N.K., et al.: On the fabrication of PDMS micromodels by rapid prototyping, and their use in two-phase flow studies. Water Resour. Res. 49(4), 2056–2067 (2013)
Kashaninejad, N., et al.: Organ-tumor-on-a-chip for chemosensitivity assay: a critical review. Micromachines 7(8), 130 (2016)
Kashaninejad, N., Shiddiky, M.J.A., Nguyen, N.-T.: Advances in microfluidics-based assisted reproductive technology: from sperm sorter to reproductive system-on-a-chip. Advanced Biosystems 2(1), 1700197 (2018)
Kawata, S., Ichimura, T., Taguchi, A., Kumamoto, Y.: Nano-Raman scattering microscopy: resolution and enhancement. Chem. Rev. 117(7), 4983–5001 (2017)
Kazemifar, F., Blois, G., Kyritsis, D.C., Christensen, K.T.: A methodology for velocity field measurement in multiphase high-pressure flow of CO2 and water in micromodels. Water Resour. Res. 51(4), 3017–3029 (2015)
Kazemifar, F., Blois, G., Kyritsis, D.C., Christensen, K.T.: Quantifying the flow dynamics of supercritical CO2–water displacement in a 2D porous micromodel using fluorescent microscopy and microscopic PIV. Adv. Water Resour. 95, 352–368 (2016)
Kazemifar, F., Kyritsis, D.C.: Experimental investigation of near-critical CO2 tube-flow and Joule-Thompson throttling for carbon capture and sequestration. Exp. Therm. Fluid Sci. 53, 161–170 (2014)
Kim, D., et al.: Reaction-based two-photon probes for in vitro analysis and cellular imaging of monoamine oxidase activity. Chem. Commun. 48(54), 6833–6835 (2012a)
Kim, H.J., Huh, D., Hamilton, G., Ingber, D.E.: Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip 12(12), 2165–2174 (2012b)
Kim, Y., Wan, J., Kneafsey, T.J., Tokunaga, T.K.: Dewetting of silica surfaces upon reactions with supercritical CO2 and brine: pore-scale studies in micromodels. Environ. Sci. Technol. 46(7), 4228–4235 (2012c)
Kim, H.N., Lee, M.H., Kim, H.J., Kim, J.S., Yoon, J.: A new trend in rhodamine-based chemosensors: application of spirolactam ring-opening to sensing ions. Chem. Soc. Rev. 37(8), 1465–1472 (2008a)
Kim, P., Kwon, K.W., Park, M.C., Lee, S.H., Kim, S.M., Suh, K.Y.: Soft lithography for microfluidics: a review. Biochip J. 2(1), 1–11 (2008b)
Kim, H.N., et al.: Rhodamine hydrazone derivatives as Hg2 + selective fluorescent and colorimetric chemosensors and their applications to bioimaging and microfluidic system. Analyst 136(7), 1339–1343 (2011)
Kim, Y.J., Kang, Y.-T., Cho, Y.-H.: Poly(ethylene glycol)-modified tapered-slit membrane filter for efficient release of captured viable circulating tumor cells. Anal. Chem. 88(16), 7938–7945 (2016)
Kim, M., Abedini, A., Lele, P., Guerrero, A., Sinton, D.: Microfluidic pore-scale comparison of alcohol- and alkaline-based SAGD processes. J. Petrol. Sci. Eng. 154, 139–149 (2017)
King, M.B.B., Mubarak, A., Kim, J.D.D., Bott, T.R.R.: The mutual solubilities of water with supercritical and liquid carbon dioxides. J. Supercrit. Fluids 5(4), 296–302 (1992)
Kjeang, E., Djilali, N., Sinton, D.: Microfluidic fuel cells: a review. J. Power Sour. 186(2), 353–369 (2009)
Laerme, F., Schilp, A., Funk, K., Offenberg, M.: Bosch deep silicon etching: improving uniformity and etch rate for advanced MEMS applications. In: 12th IEEE International Conference on Micro Electro Mechanical Systems, 1999. MEMS’99, pp. 211–216. IEEE (1999)
Lake, L.W.: Enhanced Oil Recovery. Prentice Hall, Englewood Cliffs (2014)
Le-The, H., et al.: Large-scale fabrication of free-standing and sub-[small mu]m PDMS through-hole membranes. Nanoscale 10(16), 7711–7718 (2018)
Lee, J.S., et al.: Placenta-on-a-chip: a novel platform to study the biology of the human placenta. J. Matern. Fetal Neonatal Med. 29(7), 1046–1054 (2016)
Lee Seung, G., Lee, H., Gupta, A., Chang, S., Doyle Patrick, S.: Site-selective in situ grown calcium carbonate micromodels with tunable geometry, porosity, and wettability. Adv. Func. Mater. 26(27), 4896–4905 (2016)
Lei, K.F.: Chapter 1 Materials and Fabrication Techniques for Nano- and Microfluidic Devices, Microfluidics in Detection Science: Lab-on-a-chip Technologies, pp. 1–28. The Royal Society of Chemistry, New York (2015)
Leis, A.P., Schlicher, S., Franke, H., Strathmann, M.: Optically transparent porous medium for nondestructive studies of microbial biofilm architecture and transport dynamics. Appl. Environ. Microbiol. 71(8), 4801–4808 (2005)
Lenormand, R., Touboul, E., Zarcone, C.: Numerical models and experiments on immiscible displacements in porous media. J. Fluid Mech. 189, 165–187 (1988)
Li, X., Wu, N., Rojanasakul, Y., Liu, Y.: Selective stamp bonding of PDMS microfluidic devices to polymer substrates for biological applications. Sens. Actuators A 193, 186–192 (2013)
Li, Y., Kazemifar, F., Blois, G., Christensen, K.T.: Micro-PIV measurements of multiphase flow of water and liquid CO2 in 2-D heterogeneous porous micromodels. Water Resour. Res. 53(7), 6178–6196 (2017)
Lim, L.S., et al.: Microsieve lab-chip device for rapid enumeration and fluorescence in situ hybridization of circulating tumor cells. Lab Chip 12(21), 4388–4396 (2012)
Liu, N., Aymonier, C., Lecoutre, C., Garrabos, Y., Marre, S.: Microfluidic approach for studying CO2 solubility in water and brine using confocal Raman spectroscopy. Chem. Phys. Lett. 551, 139–143 (2012)
Liu, M., Shabaninejad, M., Mostaghimi, P.: Impact of mineralogical heterogeneity on reactive transport modelling. Comput. Geosci. 104(Supplement C), 12–19 (2017)
Lu, C., Lee, L.J., Juang, Y.J.: Packaging of microfluidic chips via interstitial bonding technique. Electrophoresis 29(7), 1407–1414 (2008)
Lu, W., Guo, H., Chou, I.M., Burruss, R.C., Li, L.: Determination of diffusion coefficients of carbon dioxide in water between 268 and 473 K in a high-pressure capillary optical cell with in situ Raman spectroscopic measurements. Geochim. Cosmochim. Acta 115, 183–204 (2013)
Madou, M.J.: Fundamentals of Microfabrication: The Science of Miniaturization. CRC Press, Boca Raton (2002)
Mahoney, S.A., Rufford, T.E., Dmyterko, A.S.K., Rudolph, V., Steel, K.M.: The effect of rank and lithotype on coal wettability and its application to coal relative permeability models. In: SPE Asia Pacific Unconventional Resources Conference and Exhibition, Brisbane
Mahoney, S.A., et al.: The effect of rank, lithotype and roughness on contact angle measurements in coal cleats. Int. J. Coal Geol. 179, 302–315 (2017)
Martin, M.M., Lindqvist, L.: The pH dependence of fluorescein fluorescence. J. Lumin. 10(6), 381–390 (1975)
Martínez-Máñez, R., Sancenón, F.: Fluorogenic and chromogenic chemosensors and reagents for anions. Chem. Rev. 103(11), 4419–4476 (2003)
Martinez, A.W., Phillips, S.T., Whitesides, G.M., Carrilho, E.: Diagnostics for the developing world: microfluidic paper-based analytical devices. Anal. Chem. 82(1), 3–10 (2009)
McDonald, J.C., et al.: Fabrication of microfluidic systems in poly(dimethylsiloxane). Electrophoresis 21(1), 27–40 (2000)
Meinhart, C.D., Wereley, S.T., Santiago, J.G.: PIV measurements of a microchannel flow. Exp. Fluids 27(5), 414–419 (1999)
Mela, P., et al.: Monolayer-functionalized microfluidics devices for optical sensing of acidity. Lab Chip 5(2), 163–170 (2005)
Moebius, F., Or, D.: Interfacial jumps and pressure bursts during fluid displacement in interacting irregular capillaries. J. Colloid Interface Sci. 377(1), 406–415 (2012)
Moebius, F., Or, D.: Pore scale dynamics underlying the motion of drainage fronts in porous media. Water Resour. Res. 50(11), 8441–8457 (2014)
Moghadas, H., Saidi, M.S., Kashaninejad, N., Kiyoumarsioskouei, A., Nguyen, N.-T.: Fabrication and characterization of low-cost, bead-free, durable and hydrophobic electrospun membrane for 3D cell culture. Biomed. Microdevice 19(4), 74 (2017a)
Moghadas, H., Saidi, M.S., Kashaninejad, N., Nguyen, N.-T.: Challenge in particle delivery to cells in a microfluidic device. Drug Deliv. Transl. Res. (2017b). https://doi.org/10.1007/s13346-017-0467-3
Moghadas, H., Saidi, M.S., Kashaninejad, N., Nguyen, N.-T.: A high-performance polydimethylsiloxane electrospun membrane for cell culture in lab on a chip. Biomicrofluidics 12(2), 024117 (2018)
Mohammadzadeh, O., Chatzis, I.: Analysis of the heat losses associated with the SAGD visualization experiments. J. Petrol. Explor. Prod. Technol. 6(3), 387–400 (2016)
Mohammadzadeh, O., Rezaei, N., Chatzis, I.: Pore-level investigation of heavy oil and Bitumen recovery using solvent—aided steam assisted gravity drainage (SA-SAGD) process. Energy Fuels 24(12), 6327–6345 (2010)
Moraes, C., Mehta, G., Lesher-Perez, S.C., Takayama, S.: Organs-on-a-chip: a focus on compartmentalized microdevices. Ann. Biomed. Eng. 40(6), 1211–1227 (2012)
Morais, S., Diouf, A., Lecoutre, C., Bernard, D., Garrabos, Y., Marre, S.: Geological labs on chip-new tools for investigating key aspects of CO2 geological storage. In: The Third Sustainable Earth Sciences Conference and Exhibition (2015)
Morais, S., et al.: Monitoring CO2 invasion processes at the pore scale using geological labs on chip. Lab Chip 16(18), 3493–3502 (2016)
Morin, B., Liu, Y., Alvarado, V., Oakey, J.: A microfluidic flow focusing platform to screen the evolution of crude oil-brine interfacial elasticity. Lab Chip 16(16), 3074–3081 (2016)
Moshksayan, K., et al.: Spheroids-on-a-chip: recent advances and design considerations in microfluidic platforms for spheroid formation and culture. Sens. Actuators B Chem. 263, 151–176 (2018)
Myers, D.R., et al.: Endothelialized microfluidics for studying microvascular interactions in hematologic diseases. J. Vis. Exp. (JoVE) 64, 3958 (2012)
Nan, Z., et al.: Manufacturing microstructured tool inserts for the production of polymeric microfluidic devices. J. Micromech. Microeng. 25(9), 095005 (2015)
Nguyen, C., Kothamasu, R., He, K., Xu, L.: Low-Salinity Brine Enhances Oil Production in Liquids-Rich Shale Formations. Society of Petroleum Engineers, London (2015)
Nguyen, N.-T., Hejazian, M., Ooi, C.H., Kashaninejad, N.: Recent advances and future perspectives on microfluidic liquid handling. Micromachines 8, 186 (2017)
Nguyen, N.-T., Shaegh, S.A.M., Kashaninejad, N., Phan, D.-T.: Design, fabrication and characterization of drug delivery systems based on lab-on-a-chip technology. Adv. Drug Deliv. Rev. 65(11–12), 1403–1419 (2013)
Nieskens, T.T., Wilmer, M.J.: Kidney-on-a-chip technology for renal proximal tubule tissue reconstruction. Eur. J. Pharmacol. 790, 46–56 (2016)
Nolan, E.M., Lippard, S.J.: Tools and tactics for the optical detection of mercuric ion. Chem. Rev. 108(9), 3443–3480 (2008)
Nordbotten, J.M., Celia, M.A., Bachu, S.: Injection and storage of CO2 in deep saline aquifers: analytical solution for CO2 plume evolution during injection. Transp. Porous Media 58(3), 339–360 (2005)
Oh, Y.S., Jo, H.Y., Ryu, J.-H., Kim, G.-Y.: A microfluidic approach to water–rock interactions using thin rock sections: Pb and U sorption onto thin shale and granite sections. J. Hazard. Mater. B 324, 373–381 (2017)
Ohno, K.I., Tachikawa, K., Manz, A.: Microfluidics: applications for analytical purposes in chemistry and biochemistry. Electrophoresis 29(22), 4443–4453 (2008)
Olsen, M.G., Adrian, R.J.: Out-of-focus effects on particle image visibility and correlation in microscopic particle image velocimetry. Exp. Fluids 29(7), S166–S174 (2000)
Pacala, S., Socolow, R.: Stabilization wedges: solving the climate problem for the next 50 years with current technologies. Science 305(5686), 968–972 (2004)
Paguirigan, A.L., Beebe, D.J.: Microfluidics meet cell biology: bridging the gap by validation and application of microscale techniques for cell biological assays. BioEssays 30(9), 811–821 (2008)
Pei, H., Zhang, G., Ge, J., Jin, L., Ma, C.: Potential of alkaline flooding to enhance heavy oil recovery through water-in-oil emulsification. Fuel 104, 284–293 (2013)
Pensabene, V., et al.: Ultrathin polymer membranes with patterned, micrometric pores for organs-on-chips. ACS Appl. Mater. Interfaces 8(34), 22629–22636 (2016)
Porter, M.L., et al.: Fundamental Investigation of Gas Injection in Microfluidic Shale Fracture Networks at Geologic Conditions. American Rock Mechanics Association, New York (2015a)
Porter, M.L., et al.: Geo-material microfluidics at reservoir conditions for subsurface energy resource applications. Lab Chip 15, 4044–4053 (2015b)
Prodanov, L., et al.: Long-term maintenance of a microfluidic 3D human liver sinusoid. Biotechnol. Bioeng. 113(1), 241–246 (2016)
Qin, N., Wen, J.Z., Ren, C.L.: Highly pressurized partially miscible liquid–liquid flow in a micro-T-junction. I. Exp. Obser. Phys. Rev. E 95(4), 043110 (2017)
Raffel, M., Willert, C.E., Wereley, S.T., Kompenhans, J.: Particle Image Velocimetry: A Practical Guide. Springer, Berlin (2013)
Rangel-German, E., Kovscek, A.: A micromodel investigation of two-phase matrix-fracture transfer mechanisms. Water Resour. Res. 42(3), 1 (2006)
Rindfleisch, F., DiNoia, T.P., McHugh, M.A.: Solubility of polymers and copolymers in supercritical CO2. J. Phys. Chem. 100(38), 15581–15587 (1996)
Rodríguez, S.J., Bishop, P.L.: Three-dimensional quantification of soil biofilms using image analysis. Environ. Eng. Sci. 24(1), 96–103 (2007)
Sackmann, E.K., Fulton, A.L., Beebe, D.J.: The present and future role of microfluidics in biomedical research. Nature 507(7491), 181–189 (2014)
Santiago, J.G., Wereley, S.T., Meinhart, C.D., Beebe, D.J., Adrian, R.J.: A particle image velocimetry system for microfluidics. Exp. Fluids 25(4), 316–319 (1998)
Sato, Y., et al.: Solubilities and diffusion coefficients of carbon dioxide and nitrogen in polypropylene, high-density polyethylene, and polystyrene under high pressures and temperatures. Fluid Phase Equilib. 162(1–2), 261–276 (1999)
Schmidt, M.A.: Wafer-to-wafer bonding for microstructure formation. Proc. IEEE 86(8), 1575–1585 (1998)
Schwartz, G., Schaible, P.: Reactive ion etching of silicon. J. Vac. Sci. Technol. 16(2), 410–413 (1979)
Seah, Y.F.S., Hu, H., Merten, C.A.: Microfluidic single-cell technology in immunology and antibody screening. Mol. Aspects Med. 59, 47–61 (2017)
Seah, Y.F.S., Hu, H., Merten, C.A.: Microfluidic single-cell technology in immunology and antibody screening. Mol. Aspects Med. 59, 47–61 (2018)
Sedaghat, M., Mohammadzadeh, O., Kord, S., Chatzis, I.: Heavy oil recovery using ASP flooding: a pore-level experimental study in fractured five-spot micromodels. Can. J. Chem. Eng. 94(4), 779–791 (2016)
Sell, A., Fadaei, H., Kim, M., Sinton, D.: Measurement of CO2 diffusivity for carbon sequestration: a microfluidic approach for reservoir-specific analysis. Environ. Sci. Technol. 47(1), 71–78 (2013)
Shirota, H., Castner Jr., E.W.: Solvation in highly nonideal solutions: a study of aqueous 1-propanol using the coumarin 153 probe. J. Chem. Phys. 112(5), 2367 (2000)
Shiu, P.P., Knopf, G.K., Ostojic, M., Nikumb, S.: Rapid fabrication of tooling for microfluidic devices via laser micromachining and hot embossing. J. Micromech. Microeng. 18(2), 025012 (2008)
Sieben, V., Kharrat, A.M., Mostowfi, F.: Novel measurement of asphaltene content in oil using microfluidic technology. In: SPE Annual Technical Conference and Exhibition. Society of Petroleum Engineers, New Orleans (2013)
Silverio, V., de Freitas, S.C.: Microfabrication Techniques for Microfluidic Devices, Complex Fluid-Flows in Microfluidics, pp. 25–51. Springer, Berlin (2018)
Singh, R., et al.: Real rock-microfluidic flow cell: a test bed for real-time in situ analysis of flow, transport, and reaction in a subsurface reactive transport environment. J. Contam. Hydrol. 204, 28–39 (2017)
Singh, R., et al.: Metabolism-induced CaCO3 biomineralization during reactive transport in a micromodel: implications for porosity alteration. Environ. Sci. Technol. 49(20), 12094–12104 (2015)
Sinton, D.: Energy: the microfluidic frontier. Lab Chip 14(17), 3127–3134 (2014)
Song, W., de Haas, T.W., Fadaei, H., Sinton, D.: Chip-off-the-old-rock: the study of reservoir-relevant geological processes with real-rock micromodels. Lab Chip 14(22), 4382–4390 (2014)
Song, W., Kovscek, A.R.: Functionalization of micromodels with kaolinite for investigation of low salinity oil-recovery processes. Lab Chip 15(16), 3314–3325 (2015)
Song, W., Kovscek, A.R.: Direct visualization of pore-scale fines migration and formation damage during low-salinity waterflooding. J. Nat. Gas Sci. Eng. 34, 1276–1283 (2016)
Stephan, K., et al.: Fast prototyping using a dry film photoresist: microfabrication of soft-lithography masters for microfluidic structures. J. Micromech. Microeng. 17(10), N69 (2007)
Stevenson, J.T.M., Gundlach, A.M.: The application of photolithography to the fabrication of microcircuits. J. Phys. E: Sci. Instrum. 19(9), 654 (1986)
Syed, A.H., et al.: A combined method for pore-scale optical and thermal characterization of SAGD. J. Petrol. Sci. Eng. 146, 866–873 (2016)
Tan, S.H., Nguyen, N.-T., Chua, Y.C., Kang, T.G.: Oxygen plasma treatment for reducing hydrophobicity of a sealed polydimethylsiloxane microchannel. Biomicrofluidics 4(3), 032204 (2010)
Tanino, Y., Zacarias-Hernandez, X., Christensen, M.: Oil/water displacement in microfluidic packed beds under weakly water-wetting conditions: competition between precursor film flow and piston-like displacement. Exp. Fluids 59(2), 35 (2018)
Trietsch, S.J., Hankemeier, T., van der Linden, H.J.: Lab-on-a-chip technologies for massive parallel data generation in the life sciences: a review. Chemometr. Intell. Lab. Syst. 108(1), 64–75 (2011)
Tropea, C., Yarin, A.L., Foss, J.F.: Springer Handbook of Experimental Fluid Mechanics. Springer, Berlin (2007)
Tsao, C.-W., DeVoe, D.L.: Bonding of thermoplastic polymer microfluidics. Microfluid. Nanofluid. 6(1), 1–16 (2009)
Unsal, E., Broens, M., Armstrong, R.T.: Pore scale dynamics of microemulsion formation. Langmuir 32(28), 7096–7108 (2016)
van der Helm, M.W., van der Meer, A.D., Eijkel, J.C., van den Berg, A., Segerink, L.I.: Microfluidic organ-on-chip technology for blood–brain barrier research. Tissue Barriers 4(1), e1142493 (2016)
Verpoorte, E., De Rooij, N.F.: Microfluidics meets MEMS. Proc. IEEE 91(6), 930–953 (2003)
Vladisavljević, G.T., Kobayashi, I., Nakajima, M.: Production of uniform droplets using membrane, microchannel and microfluidic emulsification devices. Microfluid. Nanofluid. 13(1), 151–178 (2012)
Volpatti, L.R., Yetisen, A.K.: Commercialization of microfluidic devices. Trends Biotechnol. 32(7), 347–350 (2014)
Wang, W., Chang, S., Gizzatov, A.: Toward reservoir-on-a-chip: fabricating reservoir micromodels by in situ growing calcium carbonate nanocrystals in microfluidic channels. ACS Appl. Mater. Interfaces 9(34), 29380–29386 (2017)
Wang, X., Ding, B., Li, B.: Biomimetic electrospun nanofibrous structures for tissue engineering. Mater. Today 16(6), 229–241 (2013a)
Wang, Y., et al.: Application of microfluidic technology for studying islet physiology and pathophysiology. Micro Nanosyst. 5(3), 216–223 (2013b)
Wang, Y., et al.: Experimental study of crossover from capillary to viscous fingering for supercritical CO2–water displacement in a homogeneous pore network. Environ. Sci. Technol. 47(1), 212–218 (2013c)
Warkiani, M.E., et al.: Capturing and recovering of Cryptosporidium parvum oocysts with polymeric micro-fabricated filter. J. Membr. Sci. 369(1), 560–568 (2011)
Warkiani, M.E., et al.: Slanted spiral microfluidics for the ultra-fast, label-free isolation of circulating tumor cells. Lab Chip 14(1), 128–137 (2014a)
Warkiani, M.E., et al.: An ultra-high-throughput spiral microfluidic biochip for the enrichment of circulating tumor cells. Analyst 139(13), 3245–3255 (2014b)
Webb, K.F., Teja, A.S.: Solubility and diffusion of carbon dioxide in polymers. Fluid Phase Equilib. 158–160(1), 1029–1034 (1999)
Westerweel, J.: Fundamentals of digital particle image velocimetry. Meas. Sci. Technol. 8(12), 1379 (1997)
White, C.M., et al.: Separation and capture of CO2 from large stationary sources and sequestration in geological formations—coalbeds and deep saline aquifers separation and capture of CO2 from large stationary sources and sequestration in geological formations—coalbeds. J. Air Waste Manag. Assoc. 53(6), 645–715 (2003)
Whitesides, G.: The origins and the future of microfluidics. Nature 442, 368–373 (2006a)
Whitesides, G.M.: The origins and the future of microfluidics. Nature 442(7101), 368–373 (2006b)
Wiebe, R., Gaddy, V.L.: The solubility of carbon dioxide in water at various temperatures from 12° to 40° and at pressures to 500 atmospheres. Critical phenomena. J. Am. Chem. Soc. 62(4), 815–817 (1940)
Wong, I., Ho, C.-M.: Surface molecular property modifications for poly(dimethylsiloxane)(PDMS) based microfluidic devices. Microfluid. Nanofluid. 7(3), 291–306 (2009)
Wu, B., Kumar, A., Pamarthy, S.: High aspect ratio silicon etch: a review. J. Appl. Phys. 108(5), 9 (2010)
Xu, B., et al.: High efficiency integration of three-dimensional functional microdevices inside a microfluidic chip by using femtosecond laser multifoci parallel microfabrication. Sci. Rep. 6, 19989 (2016)
Xu, R., Li, R., Huang, F., Jiang, P.: Pore-scale visualization on a depressurization-induced CO2 exsolution. Sci. Bull. 62, 795–803 (2017)
Xu, W., Ok, J.T., Xiao, F., Neeves, K.B., Yin, X.: Effect of pore geometry and interfacial tension on water-oil displacement efficiency in oil-wet microfluidic porous media analogs. Phys. Fluids 26(9), 093102 (2014)
Yadali Jamaloei, B., Kharrat, R.: Analysis of microscopic displacement mechanisms of dilute surfactant flooding in oil-wet and water-wet porous media. Transp. Porous Media 81(1), 1 (2009)
Yang, S.Y., et al.: Single-file diffusion of protein drugs through cylindrical nanochannels. ACS Nano 4(7), 3817–3822 (2010)
Yang, Y.-K., Yook, K.-J., Tae, J.: A rhodamine-based fluorescent and colorimetric chemodosimeter for the rapid detection of Hg2 + ions in aqueous media. J. Am. Chem. Soc. 127(48), 16760–16761 (2005)
Yaozhong, Z., Jea-Hyeoung, H., Likun, Z., Mark, A.S., Junghoon, Y.: Soft lithographic printing and transfer of photosensitive polymers: facile fabrication of free-standing structures and patterning fragile and unconventional substrates. J. Micromech. Microeng. 24(11), 115019 (2014)
Yoon, H., Valocchi, A.J., Werth, C.J., Dewers, T.: Pore-scale simulation of mixing-induced calcium carbonate precipitation and dissolution in a microfluidic pore network. Water Resour. Res. 48(2), W02524 (2012)
Yoon, J.-Y., Kim, B.: Lab-on-a-chip pathogen sensors for food safety. Sensors 12(8), 10713–10741 (2012)
Zarikos, I.M., Hassanizadeh, S.M., van Oosterhout, L.M., van Oordt, W.: Manufacturing a Micro-model with Integrated Fibre Optic Pressure Sensors. Transport in Porous Media, New York (2018)
Zevi, Y., Dathe, A., McCarthy, J.F., Richards, B.K., Steenhuis, T.S.: Distribution of colloid particles onto interfaces in partially saturated sand. Environ. Sci. Technol. 39(18), 7055–7064 (2005)
Zhang, C., Oostrom, M., Grate, J.W., Wietsma, T.W., Warner, M.G.: Liquid CO2 displacement of water in a dual-permeability pore network micromodel. Environ. Sci. Technol. 45(17), 7581–7588 (2011)
Zhang, J., Chen, K., Fan, Z.H.: Chapter one-circulating tumor cell isolation and analysis. In: Makowski, G.S. (ed.) Advances in Clinical Chemistry, pp. 1–31. Elsevier, Amsterdam (2016)
Zhang, Q., Karadimitriou, N.K., Hassanizadeh, S.M., Kleingeld, P.J., Imhof, A.: Study of colloids transport during two-phase flow using a novel polydimethylsiloxane micro-model. J. Colloid Interface Sci. 401, 141–147 (2013)
Zhang, W., et al.: Liquid biopsy for cancer: circulating tumor cells, circulating free DNA or exosomes? Cell. Physiol. Biochem. 41(2), 755–768 (2017)
Zhang, Y., Sanati-Nezhad, A., Hejazi, S.: Geo-material surface modification of microchips using layer-by-layer (LbL) assembly for subsurface energy and environmental applications. Lab Chip 18(2), 285–295 (2018)
Zhao, B., MacMinn, C.W., Juanes, R.: Wettability control on multiphase flow in patterned microfluidics. Proc. Natl. Acad. Sci. 113(37), 10251–10256 (2016)
Zheng, X., Mahabadi, N., Yun, T.S., Jang, J.: Effect of capillary and viscous force on CO2 saturation and invasion pattern in the microfluidic chip. J. Geophys. Res. Solid Earth 122(3), 1634–1647 (2017)
Zuo, L., Zhang, C., Falta, R.W., Benson, S.M.: Micromodel investigations of CO2 exsolution from carbonated water in sedimentary rocks. Adv. Water Resour. 53(6), 188–197 (2013)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gerami, A., Alzahid, Y., Mostaghimi, P. et al. Microfluidics for Porous Systems: Fabrication, Microscopy and Applications. Transp Porous Med 130, 277–304 (2019). https://doi.org/10.1007/s11242-018-1202-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11242-018-1202-3