Abstract
The porcine epidemic diarrhea virus (PEDV) belongs to the coronavirus family, which causes acute diarrhea in pigs with higher mortality in piglets less than 2 weeks old. The PEDV is one of the major concerns of the pig industry around the world, including Asian countries and Noth America since first identified in Europe. Currently, there is no PEDV licensed vaccine to effectively prevent this disease. This study was performed for the development of a mucosal PEDV vaccine and B subunit of cholera toxin (CTB) as a carrier was employed to surpass the tolerogenic nature of GALT and induce potent immune responses against the target antigen fused to CTB. An epitope (S1D) alone or conjugated with CTB was constructed into the tobacco chloroplasts expression vector which is controlled under the chloroplast rRNA operon promoter with T7g10 5′ UTR and the psbA 3′UTR as a terminator. The homoplastomic lines were obtained by third round screening via organogenesis from the leaf tissues which were verified by PCR with antigen and chloroplast specific primers and then confirmed by Southern blot analysis. While the expression level of the S1D alone as detected by Western blotting was approximately 0.07% of total soluble protein, the CTB-S1D fusion protein was expressed up to 1.4%. The fusion protein showed binding to the intestinal membrane GM1-ganglioside receptor, demonstrating its functionality. The result shows that the highest expression of S1D could be achieved by fusion with a stable CTB protein and chloroplast transformation. Furthermore, the CTB-S1D expressed in chloroplasts of Nicotiana tabacum cv. Maryland could be assembled to pentameric form which increases the possibility to develop a mucosal vaccine against PEDV.
Key message
This study describes a protocol for improved plant expression of a porcine epidemic diarrhea immunogen by utilisation of a CTB fusion protein and chloroplast transformation. The results show that the production of CTB fusion antigen was twenty times higher than antigen alone in transplastomic plant.
Similar content being viewed by others
Introduction
PEDV is a member of the Coronaviridae family, causing acute diarrhea, vomiting, and dehydration resulting in high mortality in piglets. This dangerous virus has caused significant economic losses to the pig farming industry all around the world. In 1971, PEDV was first identified in Europe (Cheesy and Cartwright 1978; Pensaert and De Bouck 1978) and then the outbreak of the disease became more and more problematic in many other countries around the world, such as the Czech Republic, Hungary, Korea, Japan, China, Italy, the Philippines, Vietnam, and Thailand (Song et al. 2015). Coronavirus spike (S) protein is a surface antigen (type I membrane glycoprotein composing 1383–1386 amino acids long) that are cleaved by furin-like proteases in the producer cell at the junction of the receptor binding (S1) and the membrane fusion subunit (S2) (Cavanagh 1983; Frana et al. 1985) even if most coronavirus-like PEDV and severe acute respiratory syndrome coronavirus (SARS) carry noncleaved S proteins upon release (Xiao et al. 2003). The S protein plays targetedortant role in regulating interactions with specific host cell receptor glycoproteins to mediate viral entry and is targeted for neutralizing antibodies in the host (Duarte and Laude 1994; Song and Park 2012). S1D (residues 636–789), a second neutralizing epitope of the S glycoprotein of PEDV has been proven to be a suitable candidate for development of a recombinant vaccine against PEDV (Huy et al. 2016; Sun et al. 2008).
The cholera toxin (CT) produced by Vibrio cholera is one of the most efficient known enterocyte-targeting molecules. The CT molecule consists of one A subunit with enzymatic activity and five B subunits with binding activity of the host (Fasano et al. 1991; Merritt et al. 1994). CTB has not only strong adjuvant properties, but also acts as a carrier, allowing the fusion antigen to traverse the mucosal barrier and thus improve antigen immunogenicity (Holmgren et al. 1993, 2003; Wu and Russell 1998; Yu and Langridge 2001). The expression of CTB fusion protein in chloroplasts has been shown to result in correct assembling to pentamer structure with conserved antigen domain (Davoodi-Semiromi et al. 2010; Ruhlman et al. 2007; Sixma et al. 1991).
In poor and developing countries, plant-based vaccines have the potential to be a great alternative to conventional vaccines, affordable, easy to store and administer, and safe from animal cell borne pathogens (Lal et al. 2007; Streatfield 2005; Takeyama et al. 2015). The chloroplast expression system has been increasingly used as a platform for the production of high amounts of therapeutic proteins such as enzymes, antibodies and recombinant vaccines (Daniell 2006; Daniell et al. 2005; Molina et al. 2004; Ruhlman et al. 2007). Indeed, human therapeutic protein (> 7% TSP), CTB (46% TSP), a magainin 2 analogue peptide (21% TSP) and many other recombinant pharmaceutical proteins were successfully expressed in chloroplasts, at several times higher yields than a nuclear targeted expression (Daniell et al. 2001b; DeGray et al. 2001; Staub et al. 2000). In most plant species, the chloroplast genome is derived from the cytoplasm, which is less likely to spread the gene through cross-pollination with wild relatives (Daniell 2006; Daniell et al. 2005). In addition, the chloroplast expression system showed several advantages such as the ability to express polycistrons, lacking the gene silencing by the “positional effect” of the transgene in the genome, which is an issue in nuclear transformation. Also, the expression of vaccine proteins in non-edible tobacco plants could be an advantage as it is isolated from a food stuff.
The main purpose of our work is to contribute to the development of a mucosal PEDV vaccine by the employment of a CTB fusion protein and a chloroplast expression system. The enhanced expression of antigen and the capacity of the CTB fusion protein to bind to GM1 ganglioside have been demonstrated in homoplastomic tobacco lines, opening the avenue for testing of this construct as a potential oral vaccine against PEDV (Streatfield 2005; Takeyama et al. 2015).
Materials and methods
Construction of chloroplast expression vector
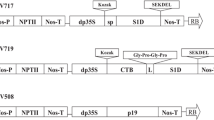
The S1D and CTB-S1D coding sequences were cloned into tobacco chloroplast expression vector under control of the tobacco rRNA promoter (Prrn) fused with the 5′ untranslated region of the bacteriophage T7 gene 10 (T7g10 5′UTR) and psbA terminator. To link T7g10 5′UTR and S1D, the fragment of S1D was released by BamHI/SacI digestion from pMYV8076 plasmid containing the S1D in the other plant expression vector and then subcloned into the intermediate vector pTKC1, resulting in pMYV8077. The T7g10 5′UTR-S1D DNA fragment digested from pMYV8077 was subcloned into tobacco chloroplast expression vector, pMYV532 to form pMYV8080 (Fig. 1). Essentially, the pMYV532 was modified from the tobacco-based chloroplast backbone vector which was kindly provided by Dr. H Daniell (University of Pensylvania, US) to insert the genes of choice. Briefly, the aminoglycoside 3′ adenyltransferase (aadA) gene controlled by 16 s rRNA (Prrn) promoter and psbA terminator were inserted between trnI and trnA flanking sequences. To construct CTB-S1D fusion gene in the chloroplast expression vector, the coding sequence of CTB from pMYV8073 were digested with BamHI restriction enzyme and subcloned into pMYV8077 to align with CTB between the T7g10. 5′UTR and S1D. The correct orientation of CTB was confirmed by PCR and DNA sequence analysis. The fragment of T7g10 5′UTR-CTB-S1D was then inserted into pMYV532 to generate chloroplast expression vector pMYV8083 (Fig. 1).
Structure of tobacco chloroplast transformation vectors for expression of S1D and CTB-S1D. The S1D or CTB-S1D coding sequences were cloned into tobacco chloroplast expression vector under the control of Prrn promoter. The trnI and trnA genes were used as flanking sequences for homologous recombination. aadA is aminoglycoside 3′ adenyltransferase gene for spectinomycin resistance. psbA-T is a terminator region of psbA gene. The 5′ untranslated region of bacteriophage T7 gene 10 (T7g10 5′UTR) as used for regulation of gene expression in tobacco chloroplast
Plant growth, biolistic transformation, and regeneration
To obtain the sterile plants, Nicotiana tabacum cv. Maryland seeds were surface-sterilized in 2% sodium hydrochloride supplemented with a few drops of Tween 20 for 10 min, washed five times with distilled water and blotted to remove the water. The seeds were germinated under sterile conditions on MS salts (Murashige and Skoog 1962) containing 3.0% sucrose and 0.8% plant agar at 25 °C. For chloroplast transformation, the leaf tissues (approximately 2 cm × 2 cm) excised from the mature tobacco plant were placed abaxial side up, on the RMOP shoot regeneration medium [Basic, MS salt, plus 0.1 ml α-naphthaleneacetic acid (NAA 1 mg/ml in 0.1 M NaOH), 1 ml 6-benzylaminopurine (BAP, 1 mg/ml in 0.1 M HCl), the 0.1 g Myo-inositol, 30 g sucrose, pH 5.8, with 1 M KOH, 7 g agar] for 1 day before performing transformations. The gold particles were coated with DNA plasmids pMYV8088 or pMYV8083 for the expression of S1D and CTB-S1D, respectively. The complexes of particle/DNA were incubated on ice for 3 min before centrifuging for 1 min to remove the supernatant. The Eppendorf centrifuge tubes containing the particle/DNA were dipped into an ultrasonic cleaner three times for 1 s each. The DNA-coated microcarriers were suspended by brief vortexing and then applied for transformation. The bombardment was conducted by the Biolistic PDS-1000/He Particle Delivery System (Bio-Rad, US) as described by the manufacturers. Briefly, the leaf tissues were bombarded twice at the 6 cm distance. After 3 days incubation on the same medium, the tobacco leaves were cut into small pieces (approximately 0.5 cm2) and transferred to selection RMOP medium supplemented with 500 mg/l spectinomycin dihydrochloride for the first round selection. The putative transformed shoots developed from the antibiotics resistant leaf tissues were rooted in the MS agar medium containing 500 mg/l of spectinomycin to develop into whole plantlet. The leaf tissues derived from the first generation plants were subjected to a second round of selection with 1000 mg/l spectinomycin and then the process was repeated until third round screening to obtain homoplastic lines.
Genomic DNA PCR analysis
Genomic DNA of untransformed and putative transgenic plants was isolated by using the ZR Genomic DNA Kit (ZYMO Research). Three specific primer sets T7g10-F/S1D-R; T7g10-F/gRB-R and 3P-F/S1D-R were used to detect the integration of target genes into the tobacco chloroplast specific genome. The amplified PCR products were observed by ethidium bromide solution after separation on 1% agarose by DNA gel electrophoresis.
Southern blot analysis
The total cellular DNA of wild-type and transplastomic tobacco lines were isolated and purified by ZR Genomic DNA kit after third rounds of regeneration on 1000 mg/l spectinomycin-containing medium. Fifteen microgram of plant DNA was digested with XhoI overnight and then separated on a 1% agarose gel by electrophoresis before transferring onto a positively charged nylon membrane (USA). The probe binding inside the trnA region (primers: 5′-TAAAGCTTTGTATCGGCTA-3′ and 5′-ATAGTATCTTGTACCTGA-3′) was generated by PCR amplification using tobacco wild-type DNA as template. The probes were radioactive phosphor (P-32) labeled according to the manufacturer’s instructions (Roche, USA). After immobilization of the DNA to the membrane, hybridization with the corresponding DIG-labeled probe and incubation of the membrane with the HRP conjugated anti-DIG antibody, the chemiluminescence signals were detected by exposure to X-ray film. The homoplastic tobacco lines for each construct (S1D and CTB-S1D) were selected for further analysis.
Protein extraction and Western blot analysis
The total soluble protein was extracted from wild-type and homoplasmy tobacco leaf tissues. The transplastomic plant leaves were ground with liquid nitrogen and homogenized with protein extraction buffer (1:2 w/v) (200 mM Tris–Cl, pH 8.0, 100 mM NaCl, 400 mM sucrose, 10 mM EDTA, 14 mM 2-mercaptoethanol, 1 mM phenylmethylsulfonyl fluoride, 0.05% Tween-20). The supernatant was recovered by double centrifugation at 13,200 RPM for 25 min at 4 °C. The concentration of total soluble protein was determined by Bradford protein assay (Bio-Rad, Inc., Hercules, CA) before conducting Western blot analysis. The 40 µg of total soluble protein (S1D and CTB-S1D) was separated in polyacrylamide gel in Tris–glycine buffer (25 mM Tris–Cl; 250 mM glycine; pH 8.3; 0.1% SDS). The pentameric and monomeric form of recombinant CTB fusion proteins was detected under non-reducing and reducing condition, respectively. The separated proteins were transferred to Hybond C membranes (Promega, USA) by wet electroblotting (Bio-Rad, USA) at 150 mA for 3 h. The non-specific binding of antibodies was blocked by 10% non-fat milk powder in TBST buffer (Tris-buffered saline with 0.05% Tween 20) at room temperature for 2 h. Subsequently, the membranes were incubated with diluted anti-S1D or anti-CTB (1:5000) polyserum in TBST buffer containing 5% non-fat dry milk at 37 °C for 2 h. The membranes were washed thrice and incubated again with a diluted anti-mouse (S1D detection) or anti-rabbit (CTB detection) IgG conjugated to alkaline phosphatase (1:7000) in TBST buffer with 5% non-fat dry milk at 37 °C for 2 h. Proteins were detected on membranes by the addition of premixed BCIP/NBT solution (Sigma, US).
Protein quantification
In order to estimate the expression level of S1D in tobacco leaf tissues, the membranes were analyzed using the AlphaEase FC software and known concentrations of recombinant S1D produced in E. coli (20, 40, and 80 ng) to generate a standard curve. The recombinant S1D protein was purified as described in our previous paper (Huy et al. 2016). Meanwhile, the GM1 ELISA analysis was used to determine the biological activity of the assembled CTB fusion protein in tobacco chloroplast. ELISA Microplates (96-wells) were incubated overnight with 3 µg/well of monosialoganglioside GM1 (Sigma, USA) in bicarbonate buffer at 4 °C. The non-specific binding was blocked with 300 µl per well of 1% BSA (Bovine Serum Albumin) in PBS buffer at 37 °C for 2 h. Five microgram of protein extracts and 60 ng of pentameric CTB protein (Sigma, USA) as a positive control were serially diluted in 100 µl per well of 0.1% BSA in PBS buffer and incubated for 2 h at 37 °C. Subsequently, the plates were washed three times with PBST buffer and incubated with 100 µl per well of rabbit anti-CT antibody (diluted 1:7000 in PBS buffer containing 0.1% BSA) at 37 °C for 2 h. The ELISA plates were again incubated with alkaline phosphatase conjugated goat anti-rabbit IgG (Promega, S372B) at a dilution of 1:7000 in PBS buffer with 0.1% BSA at 37 °C for 2 h. The color was developed by adding 100 µl per well of phosphatase substrate (Sigma S0942). The optical density was detected at a wavelength of 405 nm by ELISA reader (Sunrise, TECAN, Switzerland). The concentration of the assembled CTB-fusion protein in the tobacco chloroplast was estimated from the standard curve generated by serial dilutions of known concentrations of the commercial pentameric CTB protein (Sigma, US).
Data analysis
The data were analyzed by GraphPad Prism 7.1.0. The significant differences between the means were determined by one-way ANOVA. Values of P less than 0.05 were considered statistically significant.
Results
Construction of the expression vector and introduction into the chloroplast
The 5′ untranslated region of T7g10 gene was inserted into upstream of the CTB-S1D or S1D coding sequence and downstream of the aadA gene to enhance the high-level of foreign protein production (Molina et al. 2004; Yang et al. 2013). The trnl and trnA genes were used as flanking sequences for homologous recombination. The aadA aminoglycoside 3′ adenyltransferase gene encoded for spectinomycin resistance in tobacco plant as the selection marker. In this expression cassette, the cDNA of S1D and CTB-S1D were transcribed as a dicistron in the chloroplast due to the upstream aadA lacking a 3′ UTR, and T7g10. 5′UTR lacking promoter. The heterologous protein expression of S1D and CTB-S1D was controlled by promoter region of 16 s rRNA (Prrn) and psbA terminator (Fig. 1). These expression cassettes were integrated into Maryland (Nicotiana tabacum cv. Maryland Mammoth) genome using homologous recombination by particle bombardment method. The resistant green shoots appeared in 4–6 weeks on the selection medium containing 500–1000 µg/ml of spectinomycin sulfate and the putative homoplastomic lines were screened up to the third round by shoot organogenesis and plant regeneration (Fig. 2). The normal appearance was observed in the plants regenerated at the high concentration of spectinomycin in this study. The homoplastomic lines were transferred to the soil through the acclimation to environmental stress for 7 days and cultured to collect the seeds.
Genomic DNA PCR analysis
The homologous recombination of target gene in the tobacco plastid genome was confirmed by genomic DNA PCR analysis. The total cellular DNA extracted from leaves of putative transformed and wild-type plants were purified and used as the DNA templates. The PCR products amplified with three specific primer sets (Figs. 3a or 4a) were examined on 1% agarose gels (Figs. 3b, 4b). All plants showing the antibiotics resistance were verified in the presence of the insert in the chloroplast genome. The inserted genes amplified with T7g10-F and S1D-R primer migrated at 0.9 kb and 0.5 kb corresponding to the DNA fragments of T7g10. 5′UTR-CTB-S1D and T7g10. 5′UTR-S1D, respectively. 3P-F and gRB-R primers were used to verify the site-specific integration of the aadA along with foreign genes into chloroplast genome. The PCR DNA bands at the sizes of 3.4 kb and 2.4 kb confirmed the integration of the aadA and CTB-S1D expression cassette between the trnl and trnA flanking sequences (Fig. 3b). Also, the PCR fragments in the size of 3.0 kb and 2.0 kb confirmed the correct recombinant aadA and S1D expression cassette (Fig. 4b) in all putative transplastomic tobacco lines. There was no PCR amplification observed with any sets of primers in the wild-type plant DNA sample used as the negative control. These results show that the recombinant S1D or CTB-S1D could be integrated into the tobacco plastid genome by particle bombardment transformation method and the concentrations of 500 to 1000 µg/ml spectinomycin to select the putative transplastomic lines in Maryland tobacco plants.
Determination of transgene integration of CTB-S1D in tobacco chloroplast genome by PCR analysis. a Schematic diagram of transforming plastid sequence of CTB-S1D. The arrows are the direction of CTB-S1D transcription. b Detection of integrated genes by genomic DNA polymerase chain reaction (PCR) in transforming plants. PCR products were amplified with three specific primer sets T7g10-F/S1D-R; T7g10-F/gRB-R and 3P-F/S1D-R. Lane M: 1 kb DNA ladder; Lanes 1–5: transplastomic tobacco plants. Lane PC: DNA amplified with plasmid used as positive control in PCR analysis. Lane NC: the genomic DNA extracted from the non-transformed plant used as a negative control in PCR
Determination of transgene integration of S1D in tobacco chloroplast genome by PCR analysis. a Schematic diagram of transforming plastid sequence of S1D. The arrows are the direction of S1D transcription. b Detection of integrated genes by genomic DNA polymerase chain reaction (PCR) in transforming plants. PCR productions were amplified with three specific primer pairs T7g10-F/S1D-R; T7g10-F/gRB-R and 3P-F/S1D-R. Lane M: 1 kb DNA ladder; Lanes 1–5: transplastomic tobacco plants. Lane PC: DNA amplified with plasmid used as positive control in PCR analysis. Lane NC: the genomic DNA extracted from the non-transformed plant used as a negative control in PCR
Southern blot analysis
The transplastomic plants verified by genomic DNA PCR amplification method and screened by a third round selection were kept under sterile conditions until carrying out the detection of homoplasmy. The results of Southern blot analysis showed five transplastomic lines displaying a 5.7 kb (S1D) and 6 kb (CTB-S1D) fragments while untransformed samples yielded only a 3 kb fragment (Fig. 5). Interestingly, the homoplasmy expressing CTB-S1D was detected already in the second round screen. This outcome demonstrated that homoplasmy of both S1D and CTB-S1D has been successfully achieved in the tobacco selected with the 500 to 1000 µg/mL spectinomycin.
Southern blot analysis of WT and third-round selection transgenic plants. a The untransformed (negative control) and transformed tobacco chloroplast genomes. The genomic DNA was completely cut by XhoI and hybridized with the DNA fragment (0.7 kb) containing the flanking regions of homologous recombination. b, c Southern blot results of untransformed tobacco, Maryland (Lane WT); transgenic lines (Lanes 1–5). The WT plant chloroplast genome as negative control generated a 3 kb fragment, whereas transgenic tobacco chloroplast genomes generated a 5.7 kb fragment (S1D, fig. b) and a 6 kb fragment (CTB-S1D, fig. c)
Immunoblot analysis
Homoplasmic tobacco lines and untransformed plant leaves were collected and stored at − 70 °C in a clean Ziploc bag before performing protein extraction. A protocol for chloroplast protein extraction was described by Verma et al. (2008). Transgenic tobacco leaves (100 mg) were finely milled in liquid nitrogen before adding 200 µl freshly prepared PEB protein extract buffer (100 mM sodium chloride, 10 mM EDTA, pH 7.5, 200 mM Tris–HCl, pH 7.5, 0.05% v/v Tween-20, 0.1% wt/v SDS, 14 mM β-mercaptoethanol, 200 mM sucrose and 2 mM PMSF) for protein extraction. Supernatant was recovered after double centrifugation at 14,000 RPM for 10 min at 4 °C. The protein extracts were stored at − 70 °C for further experiments. The expression of CTB-S1D and S1D was detected by Western blot analysis under both non-reducing and reducing conditions. Target proteins were produced and folded correctly in chloroplasts and they lacked glycosylation. Under non-reducing conditions, the pentameric CTB-S1D fusion protein without glycosylation site presented a band of approximately 150 kDa when detected with anti-S1D and anti-CT antibodies (Fig. 6a and b). Under reducing conditions, the monomeric CTB-S1D fusion protein was detected at 30 kDa by the anti-S1D and anti-CT antibodies (Fig. 6c and d). Additionally, the S1D protein was also expressed in chloroplasts, showing similar molecular weight (17 kDa) to the bacterial S1D used as the positive control (Fig. 6e). There was no nonspecific antibody binding to protein extracts from untransformed tobacco as a negative control. These results showed that S1D was successfully expressed in tobacco chloroplasts, and that enhanced expression could be achieved by CTB fusion.
Western blot analysis of S1D and CTB-S1D expressed in tobacco chloroplasts. The expression of the CTB-S1D fusion protein was confirmed by detection with mouse anti-S1D (a, c) and rabbit anti-CT antibodies (b, d) under non-reducing (a, b) and reducing conditions (c, d). Lane M is pre-stained protein molecular weight standards (Thermo Scientific) for SDS-PAGE and Western blot. The expression of S1D was detected by the anti-S1D antibody under reducing conditions (e). Lane NC is chloroplast proteins extracted from the untransformed tobacco plant as negative control
GM1 binding activity and quantification of antigen proteins
The GM1 binding activity and expression levels of CTB-S1D were analyzed by GM1-ELISA (shown in Fig. 7a, b). An increase of binding-specific absorption signal was observed, showing the recombinant CTB-S1D fusion protein is biologically active and likely to have the expected pentameric structure. The amount of pentameric CTB-S1D fusion protein was calculated to be 1.4% of TSP (Fig. 7b), which was 20 times higher than S1D alone. In contrast, the amount of S1D alone in tobacco chloroplasts was calculated to be approximately 0.07% of TSP (Fig. 7c). As expected, there was no binding to protein extracts from wild-type plant used as a negative control. The high expression of CTB fusion protein and the biological activity towards mucosal surface gangliosides have both been demonstrated as a prerequisite for a potential new vaccine candidate for PEDV.
Biological activity and protein expression level of CTB-S1D and S1D produced in tobacco chloroplast. The biological activity (a), the expression level of S1D (c) and CTB-S1D fusion proteins (b) produced in the transgenic tobacco chloroplast. The GM1-ELISA was conducted in triplicate by coating the plates with GM1-ganglioside, which is a receptor for biologically active CTB. The number 1–5 are the transgenic lines of tobacco. Error bars represent the standard deviation of the mean
Discussion
PEDV causes acute diarrhea and dehydration in suckling pigs with high death rates of over 95%, leading to considerable economic losses in the swine industry. Since it was first identified in England (Takahashi et al. 1983), the severe outbreaks occurred in the South Korea (1992), China (2010–2012) and Italy (2005–2006 and 2014–2015) (Kweon et al. 1993; Huang et al. 2013; Boniotti et al. 2016). Spreading to the U.S., Canada, and Mexico in 2013 and 2014 occurred with significant genetic diversity (Vlasova et al. 2014). The annual economic loss in these countries has been estimated at approximately $900 million to $1.8 billion (Paarlberg 2014). This emerging and re-emerging swine virus epidemic requires an effective and inexpensive vaccine. As one possibility, a plant-based mucosal vaccine could be highly advantageous, as it could be administered in a crude form by feeding pigs, without expensive downstream processes such as a purification and cold chain.
PEDV belongs to enteropathogenic coronaviruses such as transmissible gastroenteritis virus (TGEV) and porcine deltacoronavirus (PDCoV). There is no PEDV licensed vaccine to effectively prevent this disease, although different types of vaccines are available against TGEV. Encouragingly, the discovery of PEDV antibody neutralizing epitopes has opened up significant opportunities for the development of recombinant PEDV vaccine candidates (Huy et al. 2011, 2012, 2016; Jianxiong et al. 2013; Kang et al. 2006; Makadiya et al. 2016; Oh et al. 2014; Oszvald et al. 2007; Pyo et al. 2009; Yang et al. 2005). Two neutralizing epitopes within spike protein, S1C (COE, 418 bp) and S1D (459 bp) were identified by Chang et al. and Sun et al. respectively (Chang et al. 2002; Sun et al. 2008) and shown to be immunogenic (Huy et al. 2012, 2016; Wang et al. 2017). In this study, the S1D epitope has been selected to yield the highest amount of antigen through the chloroplast expression system.
Bacterial A-B toxins produced by a variety of bacterial pathogens, have been utilized to increase the uptake of antigens in mucosal immune inductive sites and improve mucosal immune responses. The CT produced by Vibrio cholera is one of the most efficient known enterocyte-targeting molecules and is a typical representative of the heteromultimeric A-B toxins. The CT molecule consists of one A subunit with enzymatic activity and five B subunits with binding activity of the host. CTB is composed of five identical polypeptides (11.5 kDa each), which are assembled into a highly stable pentameric ring structure within the bacterium. CTB binds selectively to the glycosphingolipid receptor GM1-ganglioside on the intestinal epithelial cell surface. CTB was shown to function as an effective carrier and adjuvant molecule for genetically linked antigen proteins, including autoantigens and vaccine antigens. The plant-derived CTB fusion antigens are a good choice for the induction of immune response with a low amount of antigen. It has been previously demonstrated that CTB fusion protein in plant induced serum and intestinal antibodies after oral immunization (Yu and Langridge 2001). The delivery of plant-derived CTB fusion antigens into the mucosal immune system in mice has been shown previously (Kim et al. 2013). Furthermore, the native and recombinant CTB subunit was properly assembled into functional oligomers in transgenic chloroplasts (Daniell et al. 2005; DeGray et al. 2001; Ruhlman et al. 2007).
Transgenic plants have many advantages such as cost-effectiveness, good safety profile and ease of storage. However, lower levels of antigen expression in transforming plants may be insufficient to induce oral immune response against pathogens (Tien et al. 2017). Efforts to enhance the expression levels of recombinant proteins in cells and plant tissues have focused on using strong and tissue-specific promoters, codon-optimized genes and especially targeting recombinant proteins into specific organelles such as chloroplast (Daniell et al. 2001a; Lessard et al. 2002; Park et al. 2004). The chloroplast expression system has many advantages such as no transgene silencing, no position effect and high level expression of foreign protein (Daniell 2006). Therefore, chloroplast was considered a good choice host for increasing protein expression levels in transgenes. One of the approaches for increasing recombinant protein accumulation in chloroplast is boosting translation by using 5′ untranslated leader sequence (Rasala et al. 2011). Using the 5′-untranslated sequence from alfalfa mosaic virus (AMV) increased the expression levels of human respiratory syncytial virus (RSV) recombinant protein in chloroplast by 5.5 times compared to construct without the 5′-untranslated leader (Sandhu et al. 1999). Additionally, the 5′-untranslated leader sequence of tobacco mosaic virus RNA, potato virus X (PVX) RNA, rice seed storage-protein genes or bacteriophage T7g10 has also been shown to be effective in increasing transgenic protein expression level (Li et al. 2012; Liu et al. 2010; Staub et al. 2000; Yang et al. 2013). In particular, the 5′ UTR of T7g10 has been shown to be very effective in enhancing the high-level expression of target proteins (Inka Borchers et al. 2012; Kuroda and Maliga 2001; Yang et al. 2013).
The expression level of Maligaderived antigen protein is of highest relevance for determining the dose of antigen that can be delivered within protein-containing plant material. Thus, the improving expression level is necessary for developing plant-based vaccines. In this study, we have successfully improved the expression level of the CTB-S1D fusion protein in the chloroplast expression system using 5′ untranslated region of the bacteriophage T7 gene 10 (T7g10. 5′UTR) for regulation of gene expression. The bacteriophage T7g10 is not only suitable for high-level of protein production, but also the Shine-Dalgarno (SD) sequence of T7g10. 5′UTR may be an efficient mediator of translation initiation in plastid (Yang et al. 2013). The chloroplast-derived CTB-S1D fusion protein displayed biological function of a pentameric structure that could bind to GM1-ganglioside, a receptor of CTB. This result showed that the target CTB-S1D fusion protein is properly folded and the antigen binding site is conserved. Other studies have shown similar results, using either the native or recombinant CTB subunit expressed in transgenic chloroplasts (Daniell et al. 2005; DeGray et al. 2001; Ruhlman et al. 2007). The pentameric CTB-fusion protein was expressed in approximately 1.4% of TSP, which is nearly three times higher than their expression in N. benthamiana, which was estimated to be up to 0.07% of TSP (Huy et al. 2016). Furthermore, the production of CTB fusion S1D in chloroplast was 20 or 40 times higher than S1D alone expressed in stable chloroplast and transient expression in tobacco, respectively (Huy et al. 2016). This result suggested the chloroplast-derived CTB-S1D fusion protein could be considered a promising oral vaccine candidate for the prevention of enteropathogenic PEDV infection in swine and future studies will address its immunogenic potential in mice.
References
Boniotti M, Papetti A, Lavazza A, Alborali G, Sozzi E, Chiapponi C et al (2016) Porcine epidemic diarrhea virus and discovery of a recombinant swine enteric coronavirus, Italy. Emerg Infect Dis 22(1):83–87
Cavanagh D (1983) Coronavirus IBV: structural characterization of the spike protein. J Gen Virol 64(Pt 12):2577–2583. https://doi.org/10.1099/0022-1317-64-12-2577
Chang SH, Bae JL, Kang TJ, Kim J, Chung GH, Lim CW, Laude H, Yang MS, Jang YS (2002) Identification of the epitope region capable of inducing neutralizing antibodies against the porcine epidemic diarrhea virus. Mol Cells 14:295–299
Cheesy D, Cartwright S (1978) Virus-like particles associated with porcine epidemic diarrhoea. Res Vet Sci 25(2):255–256
Daniell H (2006) Production of biopharmaceuticals and vaccines in plants via the chloroplast genome. Biotechnol J 1(10):1071–1079
Daniell H, Lee S-B, Panchal T, Wiebe PO (2001a) Expression of the native cholera toxin B subunit gene and assembly as functional oligomers in transgenic tobacco chloroplasts. J Mol Biol 311(5):1001–1009
Daniell H, Lee S-B, Panchal T, Wiebe PO (2001b) Expression of the native cholera toxin B subunit gene and assembly as functional oligomers in transgenic tobacco chloroplasts1. J Mol Biol 311(5):1001–1009
Daniell H, Chebolu S, Kumar S, Singleton M, Falconer R (2005) Chloroplast-derived vaccine antigens and other therapeutic proteins. Vaccine 23(15):1779–1783
Davoodi-Semiromi A, Schreiber M, Nalapalli S, Verma D, Singh ND, Banks RK, Chakrabarti D, Daniell H (2010) Chloroplast-derived vaccine antigens confer dual immunity against cholera and malaria by oral or injectable delivery. Plant Biotechnol J 8(2):223–242
DeGray G, Rajasekaran K, Smith F, Sanford J, Daniell H (2001) Expression of an antimicrobial peptide via the chloroplast genome to control phytopathogenic bacteria and fungi. Plant Physiol 127(3):852–862
Duarte M, Laude H (1994) Sequence of the spike srotein of the porcine epidemic diarrhoea virus. J Gen Virol 75(5):1195–1200
Fasano A, Baudry B, Pumplin DW, Wasserman SS, Tall BD, Ketley JM, Kaper J (1991) Vibrio cholerae produces a second enterotoxin, which affects intestinal tight junctions. Proc Natl Acad Sci USA 88(12):5242–5246
Frana MF, Behnke JN, Sturman LS, Holmes KV (1985) Proteolytic cleavage of the E2 glycoprotein of murine coronavirus: host-dependent differences in proteolytic cleavage and cell fusion. J Virol 56(3):912–920
Holmgren J, Lycke N, Czerkinsky C (1993) Cholera toxin and cholera B subunit as oral—mucosal adjuvant and antigen vector systems. Vaccine 11(12):1179–1184
Holmgren J, Czerkinsky C, Eriksson K, Mharandi A (2003) Mucosal immunisation and adjuvants: a brief overview of recent advances and challenges. Vaccine 21:S89–S95
Huang YW, Dickerman AW, Piñeyro P, Li L, Fang L, Kiehne R, Opriessnig T, Meng XJ (2013) Origin, evolution, and genotyping of emergent porcine epidemic diarrhea virus strains in the United States. MBio 4(5):e00737–13
Huy N-X, Yang M-S, Kim T-G (2011) Expression of a cholera toxin B subunit-neutralizing epitope of the porcine epidemic diarrhea virus fusion gene in transgenic lettuce (Lactuca sativa L.). Mol Biotechnol 48(3):201–209
Huy N-X, Kim S-H, Yang M-S, Kim T-G (2012) Immunogenicity of a neutralizing epitope from porcine epidemic diarrhea virus: M cell targeting ligand fusion protein expressed in transgenic rice calli. Plant Cell Rep 31(10):1933–1942
Huy N-X, Kim M-Y, Kim T-G, Jang Y-S, Yang M-S (2016) Immunogenicity of an S1D epitope from porcine epidemic diarrhea virus and cholera toxin B subunit fusion protein transiently expressed in infiltrated Nicotiana benthamiana leaves. Plant Cell Tissue Organ Cult 127(2):369–380
Inka Borchers AM, Gonzalez-Rabade N, Gray JC (2012) Increased accumulation and stability of rotavirus VP6 protein in tobacco chloroplasts following changes to the 5′ untranslated region and the 5′ end of the coding region. Plant Biotechnol J 10(4):422–434
Jianxiong X, Zhiling T, Manlin L (2013) Cloning and expression of antigen epitopes of spike protein of porcine epidemic diarrhea virus. Guangdong J Anim Vet Sci 2:007
Kang T-J, Han S-C, Yang M-S, Jang Y-S (2006) Expression of synthetic neutralizing epitope of porcine epidemic diarrhea virus fused with synthetic B subunit of Escherichia coli heat-labile enterotoxin in tobacco plants. Protein Expr Purif 46(1):16–22
Kim M-Y, Chung N-D, Yang M-S, Kim T-G (2013) Expression of a cholera toxin B subunit and consensus dengue virus envelope protein domain III fusion gene in transgenic rice callus. Plant Cell Tissue Organ Cult 112(3):311–320
Kuroda H, Maliga P (2001) Complementarity of the 16S rRNA penultimate stem with sequences downstream of the AUG destabilizes the plastid mRNAs. Nucleic Acids Res 29(4):970–975
Kweon CH, Kwon BJ, Jung TS, Kee YJ, Hur DH, Hwang EK, Rhee JC, An SH (1993) Isolation of porcine epidemic diarrhea virus (PEDV) in Korea. Korean J Vet Res 33(2):249–254
Lal P, Ramachandran V, Goyal R, Sharma R (2007) Edible vaccines: current status and future. Indian J Med Microbiol 25(2):93
Lessard PA, Kulaveerasingam H, York GM, Strong A, Sinskey AJ (2002) Manipulating gene expression for the metabolic engineering of plants. Metab Eng 4(1):67–79
Li WJ, Dai LL, Chai ZJ, Yin ZJ, Qu LQ (2012) Evaluation of seed storage protein gene 3′-untranslated regions in enhancing gene expression in transgenic rice seed. Transgenic Res 21(3):545–553
Liu WX, Liu HL, Chai ZJ, Xu XP, Song YR, Qu LQ (2010) Evaluation of seed storage-protein gene 5′ untranslated regions in enhancing gene expression in transgenic rice seed. Theor Appl Genet 121(7):1267–1274
Makadiya N, Brownlie R, van den Hurk J, Berube N, Allan B, Gerdts V, Zakhartchouk A (2016) S1 domain of the porcine epidemic diarrhea virus spike protein as a vaccine antigen. Virol J 13(1):57
Merritt EA, Sarfaty S, Akker FVD, L’Hoir C, Martial JA, Hol WG (1994) Crystal structure of cholera toxin B-pentamer bound to receptor GM1 pentasaccharide. Protein Sci 3(2):166–175
Molina A, Hervás-Stubbs S, Daniell H, Mingo-Castel AM, Veramendi J (2004) High-yield expression of a viral peptide animal vaccine in transgenic tobacco chloroplasts. Plant Biotechnol J 2(2):141–153
Oh J, Lee K-W, Choi H-W, Lee C (2014) Immunogenicity and protective efficacy of recombinant S1 domain of the porcine epidemic diarrhea virus spike protein. Arch Virol 159(11):2977–2987
Oszvald M, Kang T-J, Tomoskozi S, Tamas C, Tamas L, Kim T-G, Yang M-S (2007) Expression of a synthetic neutralizing epitope of porcine epidemic diarrhea virus fused with synthetic B subunit of Escherichia coli heat labile enterotoxin in rice endosperm. Mol Biotechnol 35(3):215
Paarlberg PL (2014) Updated estimated economic welfare impacts of porcine epidemic diarrhea virus (PEDV). Purdue Univ Dep Agric Econ Work Pap 14(4):1–38
Park M, Kim SJ, Vitale A, Hwang I (2004) Identification of the protein storage vacuole and protein targeting to the vacuole in leaf cells of three plant species. Plant Physiol 134(2):625–639
Pensaert M, De Bouck P (1978) A new coronavirus-like particle associated with diarrhea in swine. Arch Virol 58(3):243–247
Pyo H-M, Kim I-J, Kim S-H, Kim H-S, Cho S-D, Cho I-S, Hyun B-H (2009) Escherichia coli expressing single-chain Fv on the cell surface as a potential prophylactic of porcine epidemic diarrhea virus. Vaccine 27(14):2030–2036
Rasala BA, Muto M, Sullivan J, Mayfield SP (2011) Improved heterologous protein expression in the chloroplast of Chlamydomonas reinhardtii through promoter and 5′ untranslated region optimization. Plant Biotechnol J 9(6):674–683
Ruhlman T, Ahangari R, Devine A, Samsam M, Daniell H (2007) Expression of cholera toxin B-proinsulin fusion protein in lettuce and tobacco chloroplasts–oral administration protects against development of insulitis in non-obese diabetic mice. Plant Biotechnol J 5(4):495–510
Sandhu J, Osadjan M, Krasnyanski S, Domier L, Korban S, Buetow D (1999) Enhanced expression of the human respiratory syncytial virus-F gene in apple leaf protoplasts. Plant Cell Rep 18(5):394–397
Sixma TK, Pronk SE, Kalk KH, Wartna ES, Van Zanten BA, Witholt B, Hoi WG (1991) Crystal structure of a cholera toxin-related heat-labile enterotoxin from E. coli. Nature 351(6325):371
Song D, Park B (2012) Porcine epidemic diarrhoea virus: a comprehensive review of molecular epidemiology, diagnosis, and vaccines. Virus Genes 44(2):167–175
Song D, Moon H, Kang B (2015) Porcine epidemic diarrhea: a review of current epidemiology and available vaccines. Clin Exp Vaccine Res 4(2):166–176
Staub JM, Garcia B, Graves J, Hajdukiewicz PT, Hunter P, Nehra N, Paradkar V, Schlittler M, Carroll JA, Spatola L (2000) High-yield production of a human therapeutic protein in tobacco chloroplasts. Nat Biotechnol 18(3):333–338
Streatfield SJ (2005) Plant-based vaccines for animal health. Rev Sci Technol 24(1):189–199
Sun D, Feng L, Shi H, Chen J, Cui X, Chen H, Liu S, Tong Y, Wang Y, Tong G (2008) Identification of two novel B cell epitopes on porcine epidemic diarrhea virus spike protein. Vet Microbiol 131(1):73–81
Takahashi L, Okada K, Oshima K (1983) An outbreak of swine diarrhea of a new-type associated with coronavirus-like particles in Japan. Jpn J Vet Sci 45(6):829–832
Takeyama N, Kiyono H, Yuki Y (2015) Plant-based vaccines for animals and humans: recent advances in technology and clinical trials. Ther Adv Vaccines 3 (5–6):139–154 https://doi.org/10.1177/2051013615613272
Tien N, Kim T-J, Kim T-G (2017) Viral hemorrhagic septicemia virus glycoprotein production in tobacco. Protein Expr Purif 133:170–176
Verma D, Samson NP, Koya V, Daniell H (2008) A protocol for expression of foreign genes in chloroplasts. Nat Protoc 3(4):739
Vlasova AN, Marthaler D, Wang Q, Culhane MR, Rossow KD, Rovira A, Collins J, Saif LJ (2014) Distinct characteristics and complex evolution of PEDV strains, North America, May 2013–February 2014. Emerg Infect Dis 20(10):1620
Wang X, Wang L, Huang X, Ma S, Yu M, Shi W, Qiao X, Tang L, Xu Y, Li Y (2017) Oral delivery of probiotics expressing dendritic cell-targeting peptide fused with porcine epidemic diarrhea virus COE antigen: a promising vaccine strategy against PEDV. Viruses 9(11):312
Wu H-Y, Russell MW (1998) Induction of mucosal and systemic immune responses by intranasal immunization using recombinant cholera toxin B subunit as an adjuvant. Vaccine 16(2–3):286–292
Xiao X, Chakraborti S, Dimitrov AS, Gramatikoff K, Dimitrov DS (2003) The SARS-CoV S glycoprotein: expression and functional characterization. Biochem Biophys Res Commun 312(4):1159–1164
Yang K-S, Lim S, Kwon S-Y, Kwak S-S, Kim H-S, Lee H-S (2005) Transgenic sweetpotato (Ipomoea batatas) expressing spike gene of porcine epidemic diarrhea virus. J Plant Biotechnol 32(4):263–268
Yang H, Gray BN, Ahner BA, Hanson MR (2013) Bacteriophage 5′ untranslated regions for control of plastid transgene expression. Planta 237(2):517–527
Yu J, Langridge WH (2001) A plant-based multicomponent vaccine protects mice from enteric diseases. Nature Biotechnol 19(6):548
Acknowledgements
This research was supported by the Ministry of Agriculture, Food and Rural Affairs (714001-07), and Nguyen-Quang-Duc Tien was supported by the BK21 plus program, Republic of Korea.
Author information
Authors and Affiliations
Contributions
M-YK and N-Q-DT conceived, designed and performed the overall study. N-XH performed Southern blot analysis. N-Q-DT and M-YK wrote and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Additional information
Communicated by Sergio Rosales-Mendoza.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tien, NQD., Huy, NX. & Kim, MY. Improved expression of porcine epidemic diarrhea antigen by fusion with cholera toxin B subunit and chloroplast transformation in Nicotiana tabacum. Plant Cell Tiss Organ Cult 137, 213–223 (2019). https://doi.org/10.1007/s11240-019-01562-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-019-01562-1