Abstract
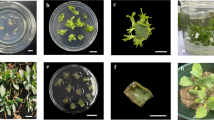
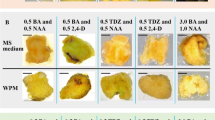
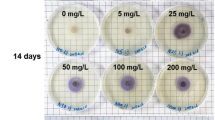
Callus tissues originating from buds of mature Scots pine (Pinus sylvestris L.) trees exhibit the typical problem of browning, which leads to degeneration and death of the tissues. The effects of medium, origin (tree and location) and endophyte infection were studied on the browning and growth of bud-derived tissue cultures. The calli growing on medium with higher kinetin content and source of organic nitrogen, and originating from the southern location grew better and exhibited less browning. Endophytic microbial cells were detected in the brown callus tissues by transmission electron microscopy. The natural endophyte infection frequency of Scots pine buds was studied and found dependent on the tree, but not on the location. A well-growing, green callus line was artificially infected by an endophytic strain of Methylobacterium extorquens, and browning was not observed on solid media compared to the uninfected control clones of the same callus. However, suspension cultures started from the infected callus died faster than cultures started from the uninfected callus. The endophyte species composition and plant genotype together with tissue culture conditions are the key factors for gaining plant tissue cultures with high regeneration capacity.





Similar content being viewed by others
References
Akiyama H, Kazuyasu F, Yamasaki O, Oono T, Iwatsuki K (2001) Antibacterial action of several tannins against Staphylococcus aureus. J Antimicrob Chemother 48:487–491. doi:10.1093/jac/48.4.487
Andersone U, Ievinsh G (2002) Changes of morphogenic competence in mature Pinus sylvestris L buds in vitro. Ann Bot (Lond) 90:293–298. doi:10.1093/aob/mcf176
Banso A, Adeyemo SO (2007) Evaluation of antibacterial properties of tannins isolated from Dichrostachys cinerea. Afr J Biotechnol 6:1785–1787
Bertani G (1951) Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol 62:293–300
Cai T, Butler L (1990) Plant regeneration from embryogenic callus initiated from immature inflorescences of several high-tannin sorghums. Plant Cell Tissue Organ Cult 20:101–110. doi:10.1007/BF00114707
Carsono N, Yoshida T (2006) Identification of callus induction potential of 15 Indonesian rice genotypes. Plant Prod Sci 9:65–70. doi:10.1626/pps.9.65
Freyermuth SK, Long RLG, Mathur S, Holland MA, Holtsford TP, Stebbins NE, Morris RO, Polacco JC (1996) Metabolic aspects of plant interaction with commensal methylotrophs. In: Lidstrom ME, Tabita RF (eds) Microbial growth on C1 compounds. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 277–284
Hohtola A (1988) Seasonal changes in explant viability and contamination of tissue cultures from mature Scots pine. Plant Cell Tissue Organ Cult 15:211–222. doi:10.1007/BF00033645
Hohtola A (1995) Somatic embryogenesis in Pinus sylvestris. In: Jain SM, Gupta PK, Newton RJ (eds) Somatic embryogenesis in woody plants, vol 3. Gymnosperms. Kluwer Academic Publishers, The Netherlands, pp 269–285
Hohtola A, Pitkänen R (1988) Browning of Scots pine Tissue Cultures. J Ult Mol S 98:336
Holland MA (1997) Occams razor applied to hormonology. Are cytokinins produced by plants? Plant Physiol 115:865–868
Holland MA, Polacco JC (1994) PPFMs and other covert contamination:is there more to plant physiology than just plant? Annu Rev Plant Physiol Plant Mol Biol 45:197–209. doi:10.1146/annurev.pp.45.060194.001213
Ivanova EG, Doronina NV, Shepelyakovskaya AO, Laman AG, Brovko FA, Trotsenko YA (2000) Facultative and obligate aerobic methylobacteria synthesize cytokinins. Microbiol 69:646–651. doi:10.1023/A:1026693805653
Ivanova EG, Doronina NV, Trotsenko YA (2001) Aerobic methylobacteria are capable of synthesizing auxins. Microbiol 70:392–397. doi:10.1023/A:1010469708107
Kalyaeva MA, Zakharchenko NS, Doronina NV, Rukavtsova EB, Ivanova EG, Alekseeva VV et al (2001) Plant growth and morphogenesis in vitro is promoted by associative methylotrophic bacteria. Russ J Plant Physiol 48:514–517. doi:10.1023/A:1016715800238
Kalyaeva MA, Ivanova EG, Doronina NV, Zakharchenko NS, Trotsenko YA, Bur’yanov YI (2003) Stimulation of wheat morphogenesis in vitro by methanotrophic bacteria. Dokl Akad Nauk 388:76–78
Keller H, Hohlfeld H, Wray V, Hahlbrock K, Scheel D, Strack D (1996) Changes in accumulation of soluble and cell wall-bound phenolics in elicitor-treated cell suspension cultures and fungus infected leaves of Solanum tuberosum. Phytochemistry 42:389–396. doi:10.1016/0031-9422(95)00866-7
Koenig RL, Morris RO, Polacco JC (2002) tRNA is the source of low-level trans-zeatin production in Methylobacterium spp. J Bacteriol 184:1832–1842. doi:10.1128/JB.184.7.1832-1842.2002
Koutsompogeras P, Kyriacou A, Zabetakis I (2006) The formation of 2, 5-dimethyl-4-hydroxy-2H-furan-3-one by cell-free extracts of Methylobacterium extorquens and strawberry (Fragaria x ananassa cv. Elsanta). Food Chem 4:1654–1661
Laukkanen H, Julkunen-Tiitto R, Hohtola A (1997) Effect of different nitrogen nutrients on the viability, protein synthesis and tannin production of Scots pine callus. Physiol Plant 100:982–988. doi:10.1111/j.1399-3054.1997.tb00026.x
Laukkanen H, Häggman H, Kontunen-Soppela S, Hohtola A (1999) Tissue browning of in vitro cultures of Scots pine: role of peroxidase and polyphenol oxidase. Physiol Plant 106:337–343. doi:10.1034/j.1399-3054.1999.106312.x
Laukkanen H, Rautiainen L, Taulavuori E, Hohtola A (2000) Changes in cellular structures and enzymatic activities during browning of Scots pine callus derived from mature buds. Tree Physiol 20:467–475
Lee CY, Whitaker RJ (1995) Enzymatic browning and its prevention. American Chemical Society, Washington, p 338
Manninen AM, Tarhanen S, Vuorinen M, Kainulainen P (2002) Comparing the variation of needle and wood terpenoids in Scots pine provenances. J Chem Ecol 28:211–228. doi:10.1023/A:1013579222600
Meir S, Philosoph-Hadas S, Aharoni N (1992) Ethylene-increased accumulation of fluorescent lipid-peroxidation products detected during senescence of parsley by a newly developed method. J Am Soc Hortic Sci 117:128–132
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Murthy BNS, Vettakkorumakankav NN, KrishnaRaj S, Odumeru J, Saxena P (1999) Characterization of somatic embryogenesis in Pelargonium x hortorum mediated by a bacterium. Plant Cell Rep 18:607–613. doi:10.1007/s002990050630
Pirttilä AM, Laukkanen H, Pospiech H, Myllylä R, Hohtola A (2000) Detection of intracellular bacteria in the buds of Scotch pine (Pinus sylvestris L.) by in situ hybridization. Appl Environ Microbiol 66:3073–3077. doi:10.1128/AEM.66.7.3073-3077.2000
Pirttilä AM, Kämäräinen T, Hirsikorpi M, Jaakola L, Hohtola A (2001) DNA isolation methods for medicinal and aromatic plants. Plant Mol Biol Rep 19:273a–273f
Pirttilä AM, Laukkanen H, Hohtola A (2002) Chitinase production in pine callus (Pinus sylvestris L.): a defense reaction against endophytes? Planta 214:848–852. doi:10.1007/s00425-001-0709-x
Pirttilä AM, Pospiech H, Laukkanen H, Myllylä R, Hohtola A (2003) Two endophytic fungi in different tissues of Scots pine buds (Pinus sylvestris L.). Microb Ecol 45:53–62. doi:10.1007/s00248-002-1038-8
Pirttilä AM, Joensuu P, Pospiech H, Jalonen J, Hohtola A (2004) Bud endophytes of Scots pine produce adenine derivatives and other compounds that affect morphology and mitigate browning of callus cultures. Physiol Plant 121:305–312. doi:10.1111/j.0031-9317.2004.00330.x
Pirttilä AM, Pospiech H, Laukkanen H, Myllylä R, Hohtola A (2005) Seasonal variations in location and population structure of endophytes in buds of Scots pine. Tree Physiol 25:289–297
Schulz B, Römmert A-K, Dammann U, Aust H-J, Strack D (1999) The endophyte-host interaction: a balanced antagonism? Mycol Res 103:1275–1283. doi:10.1017/S0953756299008540
Skoog F, Armstrong DJ (1970) Cytokinins. Annu Rev Plant Physiol 21:359–384. doi:10.1146/annurev.pp.21.060170.002043
Thomas P (2004) A three-step screening procedure for detection of covert and endophytic bacteria in plant tissue cultures. Curr Sci 87:67–72
Thomas P, Prabhakara BS, Pitchaimuthu M (2006) Cleansing the long-term micropropagated triploid watermelon cultures from covert bacteria and field testing the plants for clonal fidelity and fertility during the 7–10 year period in vitro. Plant Cell Tissue Organ Cult 85:317–329. doi:10.1007/s11240-006-9083-5
Thomas P, Kumari S, Swarna GK, Prakash DP, Dinesh MR (2007) Ubiquitous presence of fastidious endophytic bacteria in field shoots and index-negative apparently clean shoot-tip cultures of papaya. Plant Cell Rep 26:1491–1499. doi:10.1007/s00299-007-0363-2
Thomas P, Swarna GK, Patil P, Rawal RD (2008a) Ubiquitous presence of normally non- culturable endophytic bacteria in field shoot-tips of banana and their gradual activation to quiescent cultivable form in tissue cultures. Plant Cell Tissue Organ Cult 93:39–54. doi:10.1007/s11240-008-9340-x
Thomas P, Swarna GK, Roy PK, Patil P (2008b) Identification of culturable and originally non-culturable endophytic bacteria isolated from shoot tip cultures of banana cv Grand Naine. Plant Cell Tissue Organ Cult 93:55–63. doi:10.1007/s11240-008-9341-9
Visser C, Murthy BNS, Odumeru J, Saxena PK (1994) Modulation of somatic embryogenesis in hypocotyl cultures of geranium (Pelargonium x hortorum Bailey) cv.Ringo Rose by a bacterium. In Vitro Cell Dev Biol 30P:140–143
Zabetakis I (1997) Enhancement of flavour biosynthesis from strawberry (Fragaria x ananassa) callus cultures by Methylobacterium species. Plant Cell Tissue Organ Cult 50:179–183. doi:10.1023/A:1005968913237
Acknowledgments
This work was supported by Niemi Foundation and Academy of Finland (No. 105586, 118569).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pirttilä, A.M., Podolich, O., Koskimäki, J.J. et al. Role of origin and endophyte infection in browning of bud-derived tissue cultures of Scots pine (Pinus sylvestris L.). Plant Cell Tiss Organ Cult 95, 47–55 (2008). https://doi.org/10.1007/s11240-008-9413-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-008-9413-x