Abstract
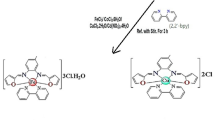
1,6-Bis(benzimidaz-2-yl)-3,4-dithiahexane ligand (L) and its mercury halide complexes were prepared and characterised. The elemental analysis, molecular conductivity, FT-Raman, FT-IR (mid, far), 1H, 13C NMR and geometry optimization in MOPAC using MNDOd parameter on CACHE, prove the existence of neutral, mononuclear and the distorted tetrahedral [Hg(L)X2] complexes. In all the three complexes, the ligand acts as a chelating bidentate, through two of the bridging sulphur atoms and together with the monodentate coordination of the two anionic halide ligands to the metal centre forming a possible 4-coordinate compounds. The antimicrobial activities of free ligand, its hydrochlorinated salt, mercury halides and the complexes are evaluated using disk diffusion method in dimethyl sulfoxide (DMSO) as well as the minimal inhibitory concentration (MIC) dilution method, against 10 bacteria. The obtained results from disk diffusion method are assessed in side-by-side comparison with those of Penicillin-g, Ampicillin, Cefotaxime, Vancomycin, Oflaxacin and Tetracyclin well-known antibacterial agents. The results from dilution procedure are compared with Gentamycin as antibacterial and Nystatin as antifungal. The antifungal activities are reported on five yeast cultures namely Candida albicans, Kluyveromyces fragilis, Rhodotorula rubra, Debaryomyces hansenii and Hanseniaspora guilliermondii, and the results are referenced with Nystatin, Ketaconazole and Clotrimazole, commercial antifungal agents. In most cases, the compounds show broad-spectrum (Gram+ & Gram− bacteria) activities that are comparatively, slightly less active or equipotent to the antibiotic and antifungal agents in the comparison tests.







Similar content being viewed by others
References
Rosenberg B (1980) Metal Ions in Biol Syst 1:1
Corey EJ, Mehrotra MM, Khan AU (1987) Science 236:68
Sadler PI (1991) Adv Inorg Chem 36:1
Bertini I, Gray HB, Lippard SJ, Valentine JS (1994) Bioinorganic chemistry. University Science Books, Sausalito
Patrick GL (2005) An introduction to medicinal chemistry, 3th edn. Oxford University Press
Giovagnini L, Marzano C, Bettio F, Fregona D (2005) J Inorg Biochem 99:39
Ma Y, Day CS, Bierbach U (2005) J Inorg Biochem 99:2013
Budakoti A, Abid M, Azam A (2006) Eur J Med Chem 41:63
Agh-Atabay NM, Dulger B, Gucin F (2005) Eur J Med Chem 40:1096
Tavman A, Agh-Atabay NM, Neshat A, Gucin F, Dulger B, Haciu D (2006) Trans Met Chem 31:194
Agh-Atabay NM, Dulger B, Gucin F (2003) Eur J Med Chem 38:875
Devereux M, Mc Cann M, Shea DO, Kelly R, Egan D, Deegan C, Kavanagh K, McKee V, Finn G (2004) J Inorg Biochem 98:1023
Aghatabay NM, Neshat A, Karabiyik T, Somer M, Haciu D, Dulger B (2007) Eur J Med Chem 42:205
Monthilal KK, Karunakaran C, Rajendran A, Murugesan R (2004) J Inorg Biochem 98:322
Irving H, Williams PJR (1953) J Chem Soc 3192
(a) Pearson RG (1963) J Am Chem Soc 85:3353; (b) Parr RG, Pearson RG (1983) J Am Chem Soc 105:7512
Addison AW, Burke PJ (1981) Heterocycl Chem 18:803
Agh-Atababy NM, Baykal A, Somer M (2004) Trans Met Chem 29:59
Performance standards for antimicrobial disk susceptibility tests (1993) Approved standard NCCLS publication M2-A5, Villanova, pp 1–32
Collins CH, Lyre PM, Grange JM (1989) Microbiological methods, 6th ed. Butterworth Co. Ltd., London
Jones RN, Barry AL, Gaven TL, Washington JA (1984) In: Lennette EH, Balows A, Shadomy WJ (eds) Manual of clinical microbiology, 4th edn. American Society for Microbiology, Washington, pp 972–977
Aghatabay NM, Somer M, Senel M, Dulger B, Gucin F (2007) Eur J Med Chem 42:1069
Yee KK, Barrow RF, Rogstad A (1974) J Chem Soc Faraday II 68:1105
Hopkins AG, Brown CW (1975) J Chem Phys 62:1598
Nakamoto K (1997) Infrared and Raman spectra of inorganic and coordination compounds, Part B, 5th edn. Wiley, p 200
Nakamoto K (1997) Infrared and Raman spectra of inorganic and coordination compounds, Part B, 5th edn. Wiley, p 199
Müller A, Jaegermann W, Enemark JH (1982) Coord Chem Rev 46:245
Chakrabarty PK, Bhattacharya S, Pierpont CG, Chakrabarty R (1992) Inorg Chem 31:3573
Guilard R, Ratti C, Tabard A, Richard P, Dubios D, Kadish KM (1990) Inorg Chem 29:2532
Müller A, Jostes R, Jaegermann W, Bhattacharyya RG (1980) Inorg Chim Acta 41:259
Müller A (1980) Analytical applications of FT-IR to molecular band biological systems. D Reidel, Dordrecht, p 257
Pearson RG (1968) J Chem Edu 45:581
Frost RL, Edwards HGM, Duong L, Kloprogge JT, Martens WN (2002) Analyst 127:293
Relf J, Cooney RP, Henneike HF (1972) Organomet Chem 39:75
Smith JH, Brill TB (1976) Inorg Chim Acta 18:225
Waters DN, Short EL, Thartwat M, Morris DFC (1973) J Mol Struct 17:389
Givan A, Loewenschuss A (1976) J Chem Phys 64:1967, ibid 65:1851
Loewenschuss A, Ron A, Schneep O (1969) J Chem Phys 50:2502
Givan A, Loewenschuss A (1978) J Chem Phys 68:2228
Nakashima S, Mishima H, Mitsuishi A (1973) Raman Spectrsc 1:325
Adams DM, Hopper MA (1971) Austr J Chem 24:885
Demiray AF (1977) Dissertation TU Clausthal Germany
Nyquist RA, Kagel RO (1971) Infrared spectra of inorganic compounds. Academic Press, New York, pp 423, 457, 475
Wells TNC, Scully P, Paravicini G, Proudfoot AEI, Payton MA (1995) Biochem 34:7896
McCutcheon AR, Ellis SM, Hancock REW, Towers GHN (1992) J Ethnopharmacol 37:213
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aghatabay, N.M., Tulu, M., Mahmiani, Y. et al. FT-Raman, FT-IR, NMR structural characterization and antimicrobial activities of 1,6-bis(benzimidazol-2-yl)-3,4-dithiahexane ligand and its Hg(II) halide complexes. Struct Chem 19, 71–80 (2008). https://doi.org/10.1007/s11224-007-9253-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-007-9253-z