Abstract
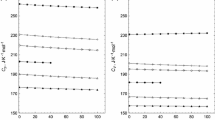
The temperature dependences of the vapor pressures of oxacyclobutan-2-one and oxacyclopentan-2-one were measured by the transpiration method. The entropies of gaseous oxacycloalkan-2-ones (lactones) were determined based on the experimental values of entropy in the condensed state, vapor pressure, and enthalpy of vaporization. Thermodynamic functions of lactones with a ring size of n = 4—8 (number of atoms in the ring) were determined by quantum chemistry and statistical physics methods in the ideal gas approximation taking into account the molar fractions of all conformers and optical isomers in the temperature range from 298.15 to 1500 K. The enthalpies of ring strain were calculated based on the enthalpies of formation.
Similar content being viewed by others
References
V. N. Emel’yanenko, S. P. Verevkin, E. N. Burakova, G. N. Roganov, M. K. Georgieva, Russ. J. Phys. Chem. A (Engl. Transl.), 2009, 83, 1271 [Zh. Fiz. Khim., 2009, 83, 1433].
V. N. Emel’yanenko, S. A. Kozlova, S. P. Verevkin, G. N. Roganov, J. Chem. Thermod., 2007, 39, 10.
V. N. Emel’yanenko, S. A. Kozlova, S. P. Verevkin, G. N. Roganov, J. Chem. Thermod., 2008, 40, 911.
V. N. Emel’yanenko, S. P. Verevkin, E. N. Burakova, G. N. Roganov, M. K. Georgieva, Russ. J. Phys. Chem. A (Engl. Transl.), 2009, 83, 598 [Zh. Fiz. Khim., 2009, 83, 697].
S. P. Verevkin, C. Schick, J. Chem. Eng. Data, 2000, 45, 946.
D. Kulikov, S. P. Verevkin, A. Heintz, J. Chem. Eng. Data, 2001, 46, 1593.
N. L. Allinger, Y. H. Yuh, J. H. Lii, J. Am. Chem. Soc., 1989, 111, 8551.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, D. J. Fox, GAUSSIAN 03 (Revision E 0.1), Gaussian Inc., Pittsburgh PA, 2007.
V. N. Emel’yanenko, V. V. Turovtsev, Yu. D. Orlov, Russ. J. Phys. Chem. A (Engl. Transl.), 2014, 88, 1472 Zh. Fiz. Khim., 2014, 88, 1307].
J. R. Durig, Spectr. Acta, 1963, 19, 1225.
S. A. Kudchadker, A. P. Kudchadker, Thermochim. Acta, 1975, 12, 11.
Z. Chen, J. van Wijngaarden, J. Mol. Spectr., 2009, 257, 164.
Z. Yu, T. Xu, T. Xing, L.-Z. Fan, F. Lian, W. Qiu, J. Power Sources, 2010, 195, 4285.
D. P. McDermott, J. Phys. Chem., 1986, 90, 2569.
S. Hesse, M. A. Suhm, Phys. Chem. Chem. Phys., 2009, 11, 11157.
V. N. Emel’yanenko, S. P. Verevkin, E. N. Stepurko, G. N. Roganov, M. K. Georgieva, Russ. J. Phys. Chem. A (Engl. Transl.), 2010, 84, 356 Zh. Fiz. Khim., 2010, 84, 418].
SDBSWeb: http:sdbs.db.aist.go.jp (National Institute of Advanced Industrial Science and Technology).
F. F. Sax, Chem. Phys., 2008, 349, 9.
L. A. Gribov, Kolebaniya molekul [Molecular Vibrations], KomKniga, Moscow, 2008, 544 pp. (in Russian).
L. A. Curtiss, P. C. Redfern, K. Raghavachari, J. Chem. Phys., 2007, 126, 84108.
L. A. Curtiss, K. Raghavachari, P. C. Redfern, J. A. Pople, J. Chem. Phys., 1997, 106, 1063.
L. A. Curtiss, P. C. Redfern, K. Raghavachari, WIREs Comput. Mol. Sci., 2011, 1, 810.
E. G. Lewars, Computational Chemistry. Dordrecht: Springer Science, 2011, 655 3.
B. Borjesson, Y. Nakase, S. Sunner, Acta Chem. Scand., 1966, 20, 803.
Yu. G. Dmitriev, K. Z. Kotovich, V. V. Kochubei, L. O. Mineravina, Vestn. L’vov. Politekhn. Inst., 1988, 221, 34 (in Russian).
N. S. Kachurina, Yu. Ya. Van-Chin-Syan, International Symposium on Calorimetry and Chemical Thermodynamics (Moscow, June 23—28, 1991), Book of Abstracts, 112.
B. V. Lebedev, A. A. Yevstropov, J. Chem. Thermod., 1983, 15, 115.
K. B. Wiberg, R. F. Waldron, J. Am. Chem. Soc., 1991, 113, 7697.
M. L. P. Leitao, H. Pilcher, Y. Meng-Yan, J. M. Brown, A. D. Conn, J. Chem. Thermod., 1990, 22, 885.
W. V. Steele, R. D. Chirico, A. Nguyen, I. A. Hossenlopp, N. K. Smith, AIChE Symp. Ser., 1989, 85, 140.
E. G. Kiparisova, B. V. Lebedev, Russ. J. Phys. Chem. A (Engl. Transl.), 1997, 71, 862 [Zh. Fiz. Khim., 1997, 71, 974].
T. S. Izmailov, N. R. Gabzalilova, Kh. M. Makhkamov, Uzbek. Khim. Zh. [Uzbek Chem. J.], 1988, 48 (in Russian).
NIST Standard Reference Database Number 69, http: webbook.nist.gov/chemistry.
V. V. Turovtsev, D. Sc. (Phys.-Math. Sci) Thesis, Tver State University, Tver, 2014, 373 pp.
N. Cohen, J. Phys. Chem. Ref. Data, 1996, 25, 1411.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 1, pp. 0082—0090, January, 2016.
Rights and permissions
About this article
Cite this article
Turovtsev, V.V., Emel’yanenko, V.N. & Orlov, Y.D. Thermodynamic functions of lactones in the gaseous state. Russ Chem Bull 65, 82–90 (2016). https://doi.org/10.1007/s11172-016-1268-4
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-016-1268-4