Abstract
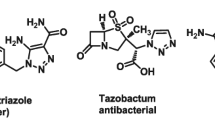
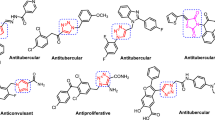
A series of spirochromene-tethered 1,2,3-triazoles (1,2,3-triazolylspirochromenes) were designed and synthesized via click-chemistry-based one-pot five-component reaction between N-propargyl isatins, malononitrile, dimedone (or 4-hydroxyl-6-methyl-2H-pyran-2-one), arylalkyl halides, and sodium azide using cellulose-supported CuI nanoparticles (Cell-CuI NPs) as heterogeneous catalyst. All synthesized compounds were screened for inhibitory activity against Mycobacterium tuberculosis H37Ra (ATCC 25177) and Mycobacterium bovis BCG (ATCC 35743), in active as well as dormant state. During screening, compounds 6h, j, l were found to exhibit promising antimycobacterial activity against M. bovis BCG, while compounds 7d, h, l showed promising antimycobacterial activity against M. tuberculosis H37Ra as well as M. bovis BCG. The active compounds were found to be noncytotoxic to three human cancer cell lines (MCF-7, HCT116, and A549). The active compounds exhibited selectivity index >10, indicating potential as antitubercular agents. The active compounds were also evaluated for in vitro antibacterial activity, with five (6h, l, 7b, f, l) showing good antibacterial activity against Gram-positive as well as Gram-negative bacteria. Compounds 6h, j, l exhibiting promising activity against M. bovis BCG can serve as good leads for further modification and optimization.







Similar content being viewed by others
References
B.H. Herzog, Respiration 65, 5 (1998)
Global Tuberculosis Control: WHO report 2014
R.P. Tripathi, N. Tewari, N. Dwivedi, V.K. Tiwari, Med. Res. Rev. 25, 93 (2005)
D.-B. Young, M.D. Perkin, K. Duncanan, C.E. Barry, J. Clin. Invest. 118, 1255 (2008)
C. Lienhardt, A. Vernon, M.C. Raviglione, Curr. Opin. Pulm. Med. 16, 186 (2010)
M.M. Sankar, J. Singh, S.C.A. Diana, S. Singh, Tuberculosis 93, 75 (2013)
M.M. Singh, Indian J. Tuberc. 54, 1 (2007)
L.T. Nao, J.J. Okogun, W.R. Folk, Nat. Prod. Rep. 30, 584 (2013)
R. Maia, C. Do, C.A.M. Fraga, Curr. Enzyme Inhib. 6, 171 (2010)
C. Viegas-Junior, A. Danuello, V. da Silva Bolzani, E.J. Barreiro, C.A.M. Fraga, Curr. Med. Chem. 14, 1829 (2007)
C.A.M. Fragae, Drug. Discov. 4, 605 (2009)
E.M. Guantai, K. Ncokazi, T.J. Egan, J. Gut, P.J. Rosenthal, P.J. Smith, K. Chibale, Bioorg. Med. Chem. 18, 8243 (2010)
K. Kurnaravel, G. Vasuki, Curr. Org. Chem. 13, 1820 (2009)
M. Syamala, Org. Prep. Proced. Int. 41, 1 (2009)
A. Dömling, W. Wang, K. Wang, Chem. Rev. 112, 3083 (2012)
V.A. Gulevich, G.A. Zhdanko, V.A.O. Romano, G.V. Nenajdenko, Chem. Rev. 110, 5235 (2010)
C. De Graaff, E. Ruijter, R.V. Orru, Chem. Soc. Rev. 41, 3969 (2012)
J.E. Biggs- Houck, A. Younai, J.T. Shaw, Curr. Opin. Chem. Biol. 14, 371 (2010)
K.S. Pandit, R.V. Kupwade, P.V. Chavan, U.V. Desai, P.P. Wadgaonkar, K.M. Kodam, ACS Sustain. Chem. Eng. 4, 3450 (2016)
K.S. Pandit, P.V. Chavan, U.V. Desai, M.A. Kulkarni, P.P. Wadgaonkar, New J. Chem. 39, 4452 (2015)
M.A. Kulkarni, V.R. Pandurangi, U.V. Desai, P.P. Wadgaonkar, C. R. Chim. 15(9), 745 (2012)
A.M. Kulkarni, K.S. Pandit, P.V. Chavan, U.V. Desai, P.P. Wadgaonkar, RSC Adv. 5, 70586 (2014)
K.S. Pandit, P.V. Chavan, U.V. Desai, M.A. Kulkarni, P.P. Wadgaonkar, New J. Chem. 39, 4452 (2015)
S.D. Mitragotri, D.M. Pore, U.V. Desai, P.P. Wadgaonkar, Catal. Commun. 9, 1822 (2008)
D.M. Pore, U.V. Desai, T.S. Thopate, P.P. Wadgaonkar, Aust. J. Chem. 60, 435 (2007)
J.F.M. da Silva, S.J. Garden, A.C. Pinto, J. Braz. Chem. Soc. 12, 273 (2001)
A.E. Medvedev, A. Clow, M. Sandler, V. Glover, Biochem. Pharmacol. 52, 385 (1996)
M. Yamazaki, E. Okuyama, Tetrahedron Lett. 22, 135 (1981)
A. Dandia, R. Singh, S. Khauria, C. Merienne, G. Morgant, A. Loupy, Bioorg. Med. Chem. 14, 2409 (2006)
J. Skommer, D. Wlodkowic, M. Matto, M. Eray, J. Pelkonen, Leuk. Res. 30, 322 (2006)
N. Yu, J.M. Aramini, M.W. Germann, Z. Huang, Tetrahedron Lett. 41, 6993 (2000)
L. Bonsignore, G. Loy, D. Secci, A. Calignano, Eur. J. Med. Chem. 28, 517 (1993)
V.V. Rostovtsev, L.G. Green, V.V. Fokin, K.B. Sharpless, Angew. Chem. 114, 2708 (2002)
V.V. Rostovtsev, L.G. Green, V.V. Fokin, K.B. Sharpless, Angew. Chem. Int. Ed. 41, 2596 (2002)
C.W. Tornøe, C. Christensen, M. Meldal, J. Org. Chem. 67, 3057 (2002)
N. Boechat, V.F. Ferreira, S.B. Ferreira, M.D.L.G. Ferreira, FdC da Silva, M.M. Bastos, M.D.S. Costa, M.C.S. Lourenço, A.C. Pinto, A.U. Krettli, A.C. Aguiar, B.M. Teixeira, N.V. da Silva, P.R.C. Martins, F.A.F.M. Bezerra, A.L.S. Camilo, G.P. da Silva, C.C.P. Costa, J. Med. Chem. 54, 5988 (2011)
S.R. Patpi, L. Pulipati, P. Yogeeswari, D. Sriram, N. Jain, B. Sridhar, R. Murthy, T. Anjana Devi, S.V. Kalivendi, S. Kantevari, J. Med. Chem. 55, 3911 (2012)
R.J. Naik, M.V. Kulkarni, K.S.R. Pai, P.G. Nayak, Chem. Biol. Drug Des. 80, 516–523 (2012)
F. Mir, S. Shaf, M.S. Zaman, N.P. Kalia, V.S. Rajput, C. Mulakayal, N. Mulakayala, I.A. Khan, M.S. Alam, Eur. J. Med. Chem. 76, 274 (2014)
D. Kumar, G. Beena, S. Khare, A.K. Kidwai, R. Tyagi, D.S.Rawat Singh, Eur. J. Med. Chem. 81, 301 (2014)
D. Addla, A. Jallapally, D. Gurram, P. Yogeeswari, D. Sriram, S. Kantevari, Bioorg. Med. Chem. Lett. 24, 1974 (2014)
M.H. Shaikh, D.D. Subhedar, L. Nawale, D. Sarkar, F.A. Kalam Khan, J.N. Sangshetti, B.B. Shingate, Med. Chem. Commun. 6, 1104 (2015)
J.M. Altimari, S.C. Hockey, H.I. Boshoff, A. Sajid, L.C. Henderso, ChemMedChem 10, 787 (2015)
T. Lee, M. Cho, S.Y. Ko, H.J. Youn, D.J. Baek, W.J. Cho, C.Y. Kang, S. Kim, J. Med. Chem. 50, 585 (2007)
Y. Xia, Z. Fan, J. Yao, Q. Liao, W. Li, F. Qu, L. Peng, Bioorg. Med. Chem. Lett. 16, 2693 (2006)
Y.-C. Duan, Y.-C. Ma, E. Zhang, X.-J. Shi, M.-M. Wang, X.-W. Ye, H.-M. Liu, Eur. J. Med. Chem. 62, 11 (2013)
Y. Zhang, Z. Lv, H.A. Zhong, D. Geng, M. Zhang, T. Zhang, Y. Li, K. Li, Eur. J. Med. Chem. 53, 356 (2012)
G.I. Wei, W. Luan, S. Wang, S. Cui, F. Li, Y. Liu, Y. Liu, M. Cheng, Org. Biomol. Chem. 13, 1507 (2015)
K. Rad-Moghadam, L. Youseftabar-Miri, Tetrahedron 67, 5693 (2011)
Z. Jamshidi, A.A. Esmaeili, J.T. Mague, J. Chem. Res. 40, 471 (2016)
S.A. Bakunov, S.M. Bakunova, T. Wenzler, M. Ghebru, K.A. Werbovetz, R. Brun, R.R. Tidwell, J. Med. Chem. 53, 254 (2010)
H. Singh, J. Sindhu, J.M. Khurana, C. Sharma, K.R. Aneja, Eur. J. Med. Chem. 77, 145 (2014)
P. Thirumurugan, D. Matosiuk, K. Jozwiak, Chem. Rev. 113, 4905 (2013)
S.G. Agalave, S.R. Maujan, V.S. Pore, Chem. Asian J. 6, 269 (2011)
R. Berg, B.F. Straub, Beilstein J. Org. Chem. 9, 2715 (2013)
P.V. Chavan, K.S. Pandit, U.V. Desai, M.A. Kulkarni, P.P. Wadgaonkar, RSC Adv. 4, 42137 (2014)
T. Mosmann, J. Immunol. Methods 65(1–2), 55 (1983)
G. Ciapetti, E. Cenni, L. Pratelli, A. Pizzoferrato, Biomaterials 14, 359 (1993)
D. Sreekanth, A. Syed, S. Sarkar, D. Sarkar, B. Santhakumari, A. Ahmad, I. Khan, J. Microbiol. Biotechnol. 19, 1342 (2009)
R. Singh, L.U. Nawale, M. Arkile, U.U. Shedbalkar, S.A. Wadhwani, D. Sarkar, B.A. Chopade, Int. J. Antimicrob. Agents 46(2), 183 (2015)
U. Singh, S. Akhtar, A. Mishra, D. Sarkar, J. Microbiol. Methods 84(2), 202 (2011)
A. Khan, D. Sarkar, J. Microbiol. Methods 73, 62 (2008)
S. Sarkar, D. Sarkar, J. Biomol. Screen. 17(7), 966 (2012)
M. Protopopova, C. Hanrahan, B. Nikonenko, R. Samala, P. Chen, J. Gearhart, L. Einck, C.A. Nacy, J. Antimicrob. Chemother. 56, 968 (2005)
M. Poggi, R. Barroso, A.J. Costa-Filho, H.B. de Barros, F. Pavan, C.Q. Leite, D. Gambino, M.H. Torre, J. Mex. Chem. Soc. 57, 198 (2013)
L.L. Gundersen, J. Nissen-Meyer, B. Spilsberg, J. Med. Chem. 45, 1383 (2002)
Acknowledgements
U. V. D. and P. V. C. thank the University Grants Commission (UGC), New Delhi, India for financial assistance [F. 43—221/2014 (SR)] and for the award of a teacher fellowship, respectively. We gratefully acknowledge Dr. K. G. Kanade, Principal, Yashavantrao Chavan Institute of Science, Satara, for encouragement.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
Experimental
General
Isatins (Aldrich), benzyl halides (Aldrich), sodium azide (S. D. Fine-Chem. Limited, Mumbai), dimedone, malononitrile, copper(I) iodide (Spectrochem, Mumbai), and microcrystalline cellulose (SRL, Mumbai) were used as received. Cell-CuI NPs as catalyst were prepared according to the procedure developed and reported by us earlier [56]. All melting points were recorded using Kumar melting point apparatus. Infrared (IR) spectra were recorded neat using a Thermo Scientific Nicolet iS10 FT-IR spectrometer. 1H nuclear magnetic resonance (NMR) (300 MHz) and 13C NMR (75.4 MHz) spectra were recorded using Bruker Avance II spectrometer. High-resolution mass spectra (HRMS) were recorded using Thermo Scientific Q-Exactive, Accela 1250 pump, instrument.
Representative procedure for one-pot synthesis of 1,2,3-triazolylspirochromenes
N-Propargyl isatin (1 mmol), malononitrile (1 mmol), dimedone (1 mmol), benzyl bromide (1 mmol), and sodium azide (1.1 mmol) were placed in a round-bottomed flask. DMF–water (1:2, 6 mL, v/v) and cellulose-CuI NPs as catalyst (0.2 g) were added, and the reaction mixture was heated at 70 °C. After reaction completion (TLC), the reaction mixture was filtered and the filter was washed with ethyl acetate (4 × 10 mL). The organic extract was washed with water and brine and dried over Na2SO4. Removal of solvent under vacuum furnished corresponding 1,4-disubstituted 1,2,3-triazolylspirochromene as solid product. The resultant solid was successively washed using a mixture of hexane–chloroform (80: 20 v/v), and dried. None of the resultant products required any further purification. The recovered catalyst was washed with acetone, dried in air, and reused for five consecutive runs.
Biological methods
Antimycobacterial activity
All synthesized compounds were screened for their in vitro activity against M. tuberculosis H37Ra (MTB) (ATCC 25177) and M. bovis BCG (ATCC 35743) using twofold dilution technique to determine the minimum inhibitory concentration (MIC). Screening against M. tuberculosis H37Ra was carried out by XTT reduction menadione assay (XRMA) by reading the absorbance at 470 nm, while screening of M. bovis BCG was carried out by nitrate reductase (NR) assay [61,62,63]. The optical density was read on a microplate reader using a 470-nm filter for XTT and a 540-nm filter for NR against blank prepared from cell-free wells. Absorbance given by cells treated with vehicle alone was taken as 100% cell growth. Initially, primary screening was done at 30, 10, and 3 µg/ml. Compounds showing 90% inhibition of bacilli at or lower than 30 µg/ml were selected for further dose–response curve analysis. All experiments were performed in triplicate, with values expressed as average ± standard deviation. MIC and IC50 values of selected compounds were calculated from dose–response curves using Origin 6 software. Percentage inhibition was calculated using the formula: % inhibition = [(control − CMP)/(control − blank)] × 100, where “control” is the activity of mycobacteria without compounds, “CMP” is the activity of mycobacteria in presence of compound, and “blank” is the activity of culture medium without mycobacteria.
Cytotoxicity and selectivity index
To check the selectivity, all compounds were assayed for their cytotoxic effects against three different cell lines (MCF-7, A549, and HCT116) by MTT assay (Table 4) [64,65,66]. The cell lines were maintained at 37 °C under 5% CO2/95% air humidified environment. The concentration range for each compound was selected as 30, 10, and 3 µg/mL. Each concentration was tested in duplicate in a single experiment. GI50 and MIC values were calculated using OriginPro software. Viability and growth in presence of test material were calculated using the following formula: % cytotoxicity = [(average absorbance of control − absorbance of compound)/(absorbance of control − absorbance of blank)] × 100, where control is the culture medium with cells and dimethyl sulfoxide (DMSO), and blank is the culture medium without cells. The selectivity index (SI) was calculated by dividing the 50% growth inhibition concentration (GI50) value for each cell line (MCF-7, A549, and HCT116) by the MIC for in vitro activity against active/dormant MTB and BCG [60].
Antibacterial activity
All bacterial cultures were first grown in Luria-Bertani (LB) medium at 37 °C at 180 RPM. Once the culture reached 1 OD, it was used for antibacterial assay. Bacterial strains E. coli (NCIM 2688), P. aeruginosa (NCIM 2036) as Gram-negative and B. subtilis (NCIM 2079), S. aureus (NCIM 2010) as Gram-positive strains were obtained from NCIM (NCL, Pune) and grown in Luria-Bertani medium. Screening was carried out by adding 0.1% 1-OD inoculated culture to each well of a 96-well plate containing the compounds to be tested and measuring the optical density at 620 nm after 8 h for Gram-negative or 12 h for Gram-positive bacteria.
Rights and permissions
About this article
Cite this article
Chavan, P.V., Pandit, K.S., Desai, U.V. et al. Click-chemistry-based multicomponent condensation approach for design and synthesis of spirochromene-tethered 1,2,3-triazoles as potential antitubercular agents. Res Chem Intermed 43, 5675–5690 (2017). https://doi.org/10.1007/s11164-017-2955-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-017-2955-y