Abstract
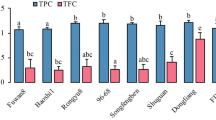
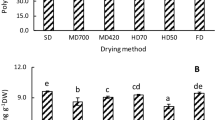
The study investigated the changes in the metabolite, antioxidant and α-glucosidase inhibitory activities of Phyllanthus niruri after three drying treatments: air, freeze and oven dryings. Water extracts and extracts obtained using different solvent ratios of ethanol and methanol (50, 70, 80 and 100 %) were compared. The relationships among the antioxidant, α-glucosidase inhibitory activity and metabolite levels of the extracts were evaluated using partial least-square analysis (PLS). The solvent selectivity was assessed based on the phytochemical constituents present in the extract and their concentrations quantitatively analyzed using high performance liquid chromatography. The freeze-dried P. niruri samples that were extracted with the mixture of ethanol or methanol with low ratio of water showed higher biological activity values compared with the other extracts. The PLS results for the ethanolic with different ratio and water extracts demonstrated that phenolic acids (chlorogenic acid and ellagic acid) and flavonoids were highly linked to strong α-glucosidase inhibitory and antioxidant activities.

Similar content being viewed by others
Abbreviations
- PLS:
-
Partial least-squares analysis
- HPLC:
-
High performance liquid chromatography
- AD:
-
Air drying
- FD:
-
Freeze drying
- OD:
-
Oven drying
- TPC:
-
Total phenolic content
- DPPH:
-
Diphenylpicrylhydrazyl
- IC50 :
-
Half maximal inhibitory concentration
- FRAP:
-
Ferric reducing antioxidant potential
References
Siddhuraju P, Becker K (2003) Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J Agric Food Chem 51:2144–2155
Markom M, Hasan M, Daud WRW, Singh H, Jahim JM (2007) Extraction of hydrolysable tannins from Phyllanthus niruri Linn.: effects of solvents and extraction methods. Sep Purif Technol 52:487–496
Sultana B, Anwar F, Ashraf M (2009) Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 14:2167–2180
Trabelsi N, Megdiche W, Ksouri R, Falleh H, Oueslati S, Soumaya B, Hajlaoui H, Abdelly C (2010) Solvent effects on phenolic contents and biological activities of the halophyte Limoniastrum monopetalum leaves. LWT-Food Sci Technol 43:632–639
Lim YY, Murtijaya J (2007) Antioxidant properties of Phyllanthus amarus extracts as affected by different drying methods. LWT-Food Sci Technol 40:1664–1669. doi:10.1016/j.lwt.2006.12.013
Chan EWC, Lim YY, Wong SK, Lim KK, Tan SP, Lianto FS, Yong MY (2009) Effects of different drying methods on the antioxidant properties of leaves and tea of ginger species. Food Chem 113:166–172
Mediani A, Abas F, Khatib A, Maulidiani H, Shaari K, Choi YH, Lajis NH (2012) 1H-NMR-based metabolomics approach to understanding the drying effects on the phytochemicals in Cosmos caudatus. Food Res Int 49:763–770
Kassuya CA, Leite DF, de Melo LV, Rehder VLG, Calixto JB (2005) Anti-inflammatory properties of extracts, fractions and lignans isolated from Phyllanthus amarus. Planta Med 71:721–726
Kumaran A, Joel Karunakaran R (2007) In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT-Food Sci Technol 40:344–352. doi:10.1016/j.lwt.2005.09.011
Patel JR, Tripathi P, Sharma V, Chauhan NS, Dixit VK (2011) Phyllanthus amarus: ethnomedicinal uses, phytochemistry and pharmacology: a review. J Ethnopharmacol 164:286–313
Lee SY, Mediani A, Nur Ashikin AH, Azliana ABS, Abas F (2014) Antioxidant and a-glucosidase inhibitory activities of the leaf and stem of selected traditional medicinal plants. Int Food Res J 21:165–174
Firuzi O, Lacanna A, Petrucci R, Marrosu G, Saso L (2005) Evaluation of the antioxidant activity of flavonoids by “ferric reducing antioxidant power” assay and cyclic voltammetry. BBA-Gen Subjects 1721:174–184
Kim J, Kwon C, Son KH (2000) Inhibition of alpha-glucosidase and amylase by luteolin, a flavonoid. Biosci Biotechnol Biochem 64:2458–2461
Mediani A, Abas F, Ping TC, Khatib A, Lajis NH (2012) Influence of growth stage and season on the antioxidant constituents of Cosmos caudatus. Plant Foods Hum Nutr 67:344–350
Jimenez P, Cabrero P, Basterrechea JE, Tejero J, Cordoba-Diaz D, Cordoba-Diaz M, Girbes T (2014) Effects of short-term heating on total polyphenols, anthocyanins, antioxidant activity and lectins of different parts of dwarf elder (Sambucus ebulus L.). Plant Foods Hum Nutr 69:168–174
Chethan S, Sreerama Y, Malleshi N (2008) Mode of inhibition of finger millet malt amylases by the millet phenolics. Food Chem 111:187–191
Hsu B, Coupar IM, Ng K (2006) Antioxidant activity of hot water extract from the fruit of the Doum palm, Hyphaene thebaica. Food Chem 98:317–328
Javadi N, Abas F, Hamid AA, Simoh S, Shaari K, Ismail IS, Mediani A, Khatib A (2014) GC-MS-based metabolite profiling of Cosmos caudatus leaves possessing alpha-glucosidase inhibitory activity. J Food Sci 79:C1130–C1137
Cheng Z, Su L, Moore J, Zhou K, Luther M, Yin JJ, Yu LL (2006) Effects of postharvest treatment and heat stress on availability of wheat antioxidants. J Agric Food Chem 54:5623–5629
Iqbal S, Bhanger M (2006) Effect of season and production location on antioxidant activity of Moringa oleifera leaves grown in Pakistan. J Food Compos Anal 19:544–551
Deutschländer M, Van de Venter M, Roux S, Louw J, Lall N (2009) Hypoglycaemic activity of four plant extracts traditionally used in South Africa for diabetes. J Ethnopharmacol 124:619–624
Matsui T, Ebuchi S, Fujise T, Abesundara KJ, Doi S, Yamada H, Matsumoto K (2004) Strong antihyperglycemic effects of water-soluble fraction of Brazilian propolis and its bioactive constituent, 3, 4, 5-tri-O-caffeoylquinic acid. Biol Pharm Bull 27:1797–1803
Kang B, Racicot K, Pilkenton SJ, Apostolidis E (2014) Evaluation of the in vitro anti-hyperglycemic effect of Cinnamomum cassia derived phenolic phytochemicals, via carbohydrate hydrolyzing enzyme inhibition. Plant Foods Hum Nutr 69:155–160
Okoli C, Obidike IC, Ezike A, Akah P, Salawu O (2011) Studies on the possible mechanisms of antidiabetic activity of extract of aerial parts of Phyllanthus niruri. Pharm Biol 49:248–255. doi:10.3109/13880209.2010.501456
Shetty A, Rashmi R, Rajan M, Sambaiah K, Salimath P (2004) Antidiabetic influence of quercetin in streptozotocin-induced diabetic rats. Nutr Res 24:373–381
Wansi JD, Lallemand M, Chiozem DD, Toze FAA, Mbaze LM, Naharkhan S, Iqbal MC, Tillequin F, Wandji J, Fomum ZT (2007) α-Glucosidase inhibitory constituents from stem bark of Terminalia superba (Combretaceae). Phytochemistry 68:2096–2100
Maisuthisakul P, Pasuk S, Ritthiruangdej P (2008) Relationship between antioxidant properties and chemical composition of some Thai plants. J Food Compos Anal 21:229–240
Coman C, Ruginǎ OD, Socaciu C (2012) Plants and natural compounds with antidiabetic action. Not Bot Horti Agrobo 40:314–325
Pandey KB, Rizvi SI (2009) Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev 2:270–278
Acknowledgments
The authors wish to thank Universiti Putra Malaysia and Ministry of Agriculture (MOA) for the NRGS grant provided (NH0612D008).
Conflict of interest
The authors declare that they have no conflict of interest. This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mediani, A., Abas, F., Khatib, A. et al. Relationship Between Metabolites Composition and Biological Activities of Phyllanthus niruri Extracts Prepared by Different Drying Methods and Solvents Extraction. Plant Foods Hum Nutr 70, 184–192 (2015). https://doi.org/10.1007/s11130-015-0478-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-015-0478-5