Abstract
The dependence on temperature of tryptophan fluorescence lifetime in trimeric photosystem I (PSI) complexes from cyanobacteria Synechocystis sp. PCC 6803 during the heating of pre-frozen to − 180 °C in the dark or in the light-activated preparations has been studied. Fluorescence lifetime in samples frozen in the light was longer than in samples frozen in the dark. For samples in 65% glycerol at λreg = 335 nm and at 20 °C, the lifetime of components were as follows: τ1 ≈ 1.2 ns, τ2 ≈ 4.9 ns, and τ3 ≈ 20 ns. The contribution of the first component was negligible. To analyze the contribution of components 2 and 3 derived from frozen-thawed samples, two temperature ranges from − 180 to − 90 °C and above − 90 °C are considered. In doing so, the contributions of these components appear antiphase course to each other. The dependence on temperature of these contributions is explained by the influence of the microconformational protein dynamics on the tryptophan fluorescence lifetime. In the present work, a comparative analysis of temperature-dependent conformational dynamics and electron transfer in cyanobacterial PSI (Schlodder et al., in Biochemistry 37:9466–9476, 1998) and Rhodobacter sphaeroides reaction center complexes (Knox et al., in J Photochem Photobiol B 180:140–148, 2018) was also carried out.

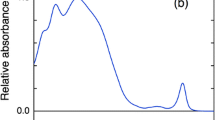
(Reproduced with permission from Knox et al. 2018)



(Reproduced with permission from Schlodder et al. 1998)
Similar content being viewed by others
Abbreviations
- RC:
-
Reaction center
- PSI, PSII:
-
Photosystem I and II, respectively
- P870, P700:
-
Photoactive pigments of bacterial RC and PSI
- Q A, Q B :
-
Primary and secondary quinone acceptors, respectively
- F X, F A, F B :
-
Iron–sulfur clusters
- Chl:
-
Chlorophyll
- BChl:
-
Bacteriochlorophyll
- DAS:
-
Decay associated spectra
- τ fl :
-
Fluorescence lifetime
- τ av :
-
Average fluorescence lifetime
- LHC:
-
Light harvesting complex
References
Albani JR (2011) Relation between proteins tertiary structure, tryptophan fluorescence lifetimes and tryptophan So → 1Lb and So → 1La transitions, Studies on α1-acid glycoprotein and β-lactoglobulin. J Fluoresc 21:1301–1309
Arnon JR (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in beta vulgaris. Plant Physiol 24:1–15
Baymann F, Brugna M, Muhlenhoff U, Nitschke W (2001) Daddy, where did (PS)I come from? Biochim Biophys Acta 1507:291–310
Burstein EA (1983) The intrinsic luminescence of proteins is a method for studies of the fast structural dynamics. Mol Biol (Moscow) 17:455–467
Callis PR, Vivian JT (2003) Understanding the variable fluorescence quantum yield of tryptophan in proteins using QM-MM simulations. Quenching by charge transfer to the peptide backbone. Chem Phys Lett 369:409–414
Chen Y, Barkley MD (1998) Toward understanding tryptophan fluorescence in proteins. Biochemistry 3:9976–9982
Fathir I, Mori T, Nogi T, Kobayashi M, Miki K, Nozawa T (2001) Structure of the H subunit of the photosynthetic reaction center from the thermophilic purple sulfur bacterium Thermochromatium tepidum complex. Eur J Biochem 268:2652–2657
Godik VI, Blankenship RE, Causgrove TP, Woodbury N (1993) Time-resolved tryptophan fluorescence in photosynthetic reaction centers from Rhodobacter sphaeroides. FEBS Lett 321:229–232
Gorokhov VV, Knox PP, Korvatovskiy BN, Seifullina NKh, Goryachev SN, Paschenko VZ (2017) Temperature dependence of tryptophan fluorescence lifetime in aqueous glycerol and trehalose solutions. Biochemistry 82:1269–1275
Hellings M, De Maeyer M, Verheyden S, Hao Q, Van Damme EJM, Peumans VJ, Engelborghs Y (2003) The dead-end elimination method, tryptophan rotamers and fluorescence lifetimes. Biophys J 85:1894–1902
Kleinfeld D, Okamura N, Feher G (1984) Electron-transfer kinetics in photosynthetic reaction centers cooled to cryogenic temperatures in the charge-separated state: evidence for lightinduced structural changes. Biochemistry 23:5780–5786
Knox PP, Churbanova IYu, Paschenko VZ (1998) On the retalationship between structural and dynamic properties in purple bacteria reaction centers and stabilization of photomobilized electron on quinone acceptors. In: Garab G (ed) Photosynthesis: mechanisms and effects. Kluwer Acad. Publ., Amsterdam, pp 821–824
Knox PP, Heinnickel M, Rubin AB (2004) Effect of low temperatures on photochemical activity of PS1 reaction centers from Synechocystis sp. frozen under illumination. Biochemistry 69:1399–1402
Knox PP, Krasilnikov PM, Heinnickel M, Rubin AB (2006) Kinetics of pigment–acceptor interaction induced by continuous illumination in Synechocystis sp. photosystem I preparations cooled to 160 K in the dark and light. Biophysics 51:51–56
Knox PP, Korvatovsky BN, Krasilnikov PM, Paschenko VZ, Seifullina NH, Grishanova NP, Rubin AB (2016a) Temperature dependence of protein fluorescence in Rb. sphaeroides reaction centers frozen to 80 K in the dark or on the actinic light as the indicator of protein conformational dynamics. Doklady Biochem Biophys 467:105–109
Knox PP, Lukashev EP, Korvatovskii BN, Gorokhov VV, Grishanova NP, Seyfullina NKh, Paschenko VZ, Rubin AB (2016b) A comparison of the temperature dependence of charge recombination in the ion-radical pair and tryptophan fluorescence in the photosynthetic reaction centers of Rhodobacter sphaeroides. Biophysics 61:877–884
Knox PP, Gorokhov VV, Korvatovskiy BN, Lukashev EP, Goryachev SN, Paschenko VZ, Rubin AB (2018) The effect of temperature on the dynamic state of Rb. sphaeroides reaction center proteins determined from changes in the tryptophan fluorescence lifetime and the P+Q– A recombination kinetics. J Photochem Photobiol B 180:140–148
Lakowicz JR (1999) Principles of fluorescence spectroscopy, 2nd edn. Plenum Press, New York
Liu T, Callis PR, Hesp BH, de Groot M, Buma WJ, Broos J (2005) Ionization potentials of fluoroindoles and the origin of nonexponential tryptophan fluorescence decay in proteins. J Am Chem Soc 127:4104–4113
Noks PP, Lukashev EP, Kononenko AA, Venediktov PS, Rubin AB (1977) Possible role of macromolecular components in functioning of photosynthetic reaction centers of purple bacteria. Mol Biol (Moscow) 11:835–842
Pieper J, Renger G (2009) Protein dynamics investigated by neutron scattering. Photosyn Res 102:281–293
Pieper J, Schödel R, Irrgang K-D, Voigt J, Renge G (2001) Electron-phonon coupling in solubilized LHC II complexes of green plants investigated by line-narrowing and temperature dependent fluorescence spectroscopy. J Phys Chem B 105:7115–7124
Pieper J, Hauss T, Buchsteiner A, Baczyn´ski K, Adamiak K, Lechner RE, Renger G (2007) Temperature- and hydration-dependent protein dynamics in photosystem II of green plants studied by quasielastic neutron scattering. Biochemistry 46:11398–11409
Reddy NRS, Lyle PA, Small GJ (1992) Applications of spectral hole burning spectroscopies to antenna and reaction center complexes. Photosyn Res 31:167–194
Reshetnyak YK, Burstein EA (2001) Decomposition of protein tryptophan fluorescence spectra into log-normal components. II. The statistical proof of discreteness of tryptophan classes in proteins. Biophys J 81:1710–1734
Ross JA, Jameson DM (2008) Time-resolved methods in biophysics. 8. Frequency domain fluorometry: applications to intrinsic protein fluorescence. Photochem Photobiol Sci 7:1301–1313
Schauerte JA, Gafni A (1989) Long-lived tryptophan fluorescence in phosphoglycerate mutase. Biochemistry 28:3948–3954
Schlodder E, Falkenberg K, Gergeleit M, Brettel K (1998) Temperature dependence of forward and reverse electron transfer from A1 –, the reduced secondary electron acceptor in photosystem I. Biochemistry 37:9466–9476
Semenov AYu, Kurashov VN, Mamedov MD (2011) Transmembrane charge transfer in photosynthetic reaction centers: some similarities and distinctions. J Photochem Photobiol B 104:326–332
Shen GZ, Zhao JD, Reimer SK, Antonkine ML, Cai Q, Weiland SM, Golbeck JH, Bryant DA (2002) Assembly of photosystem II. Inactivation of the rubA gene encoding a membrane-associated rubredoxin in the cyanobacterium Synechococcus sp PCC 7002 causes a loss of photosystem I activity. J Biol Chem 277:20343–20354
Szabo AG, Rayner DM (1980) Fluorescence decay of tryptophan conformers in aqueous solution. J Am Chem Soc 102:554–563
Tietz C, Jelezko F, Gerken U, Schuler S, Schubert A, Rogl H, Wrachtrup J (2001) Single molecule spectroscopy on the light-harvesting complex II of higher plants. Biophys J 81:556–562
Williams JC, Steiner LA, Feher G, Simon MI (1984) Primary structure of the L subunit of the reaction center from Rhodopseudomonas sphaeroides. Proc Natl Acad Sci USA 81:7303–7307
Wu H-M, Rätsep M, Jankowiak R, Cogdell RJ, Small GJ (1997) Comparison of the LH2 antenna complexes of Rhodopseudomonas acidophila (strain 10050) and Rhodobacter sphaeroides by high-pressure absorption, high-pressure hole burning, and temperature-dependent absorption spectroscopies. J Phys Chem B 101:7641–7653
Xu Q, Gunner MR (2001) Trapping conformational intermediate states in the reaction center protein from photosynthetic bacteria. Biochemistry 40:3232–3241
Acknowledgements
This study was supported by the Russian Foundation for Basic Research (Project No. 15-29-01167).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Knox, P.P., Korvatovskiy, B.N., Gorokhov, V.V. et al. Comparison of tryptophan fluorescence lifetimes in cyanobacterial photosystem I frozen in the light and in the dark. Photosynth Res 139, 441–448 (2019). https://doi.org/10.1007/s11120-018-0595-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-018-0595-8