Abstract
Background and aims
Ultramafic soils constitute an extreme environment for plants because of specific physico-chemical properties and the presence of Ni, Cr, and Co. We hypothesized that type of ultramafic parent rock depending on their origin affects the composition of soils and plants. Therefore, phytoavailability of metals would be higher in soil derived from serpentinized peridotite compared to serpentinite because of differences in susceptibility of minerals to weathering.
Results
Based on DTPA-CaCl2 extractions, we noted that soil derived from the serpentinized peridotite is characterized by a higher phytoavailability of Ni compared to soil derived from the serpentinite. On the contrary, plant species growing on soil derived from the serpentinite contain higher concentrations of metals.
Conclusions
Our study suggests that the metal uptake by plants is controlled by the mineral composition of parent rocks, which results from both their original magmatic composition and later metamorphic processes. Chemical extractions show that the phytoavailability of Ni and Co is higher in soil derived from the serpentinized peridotite than the serpentinite. Surprisingly, plants growing on the soil derived from the serpentinite contain higher levels of metals compared to these from the serpentinized peridotite derived soil. This contrasting behavior is due to higher abundances of Ca and Mg, not only Ni and Co, in soil derived from the serpentinized peridotite as compared to those in the soil derived from the serpentinite. Calcium and Mg are favored by plants and preferably fill the available sites, resulting in low Ni and Co intake despite their higher abundances.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ultramafic soils refer to specific soils derived from weathering of peridotites and/or serpentinites. Peridotite is an igneous rock composed mostly of olivine, whereas serpentinite is formed during serpentinization and consists of hydrous silicates, e.g. serpentine (Coleman and Jove 1992). Other minerals such as magnetite are minor in both above mentioned rocks. Ultramafic soils are characterized by several specific traits: (a) low Ca/Mg ratio; (b) deficiency of nutrients such as: N, P and K and (c) high contents of Ni, Cr, and Co (Whittaker 1954; Kruckeberg 2004; O’Dell and Claassen 2006; Kazakou et al. 2008; Oze et al. 2008). All these characteristics cause ultramafic soils to be low productivity soils. The response of vegetation to such stressful conditions was named “serpentine syndrome” by Jenny in 1980 (after Kruckeberg 2004).
One of the major challenges for plants growing on ultramafic soils is the high content of Mg at deficiency levels of Ca (Proctor 1970). Based on experiments with Agrostis genus the author proved that Mg can be toxic for plants (i.e. reduction in root growth). However, species growing on ultramafic soils are characterized by greater tolerance to high contents of Mg in soils compared with species from non-ultramafic soils. The previous research indicates that adaptation of plants for low Ca/Mg status of ultramafic soils is associated with several mechanisms: (a) selectivity, (b) tolerance to low content of Ca and high content of Mg and (c) luxury consumption of Mg (reviewed in Kazakou et al. 2008). Studies with Arctostaphylos viscida showed that selectivity in species from ultramafic soils lies in higher ability to translocation of Ca than Mg from roots to aboveground parts compared with non-ultramafic species (O’Dell et al. 2006). Furthermore, tolerance to deficiency of Ca and excess of Mg was observed in Agrostis stolonifera (Marrs and Proctor 1976). The latter mechanism, called luxury consumption, involves uptake and storage of Mg for later use (i.e. Helianthus bolanderi ssp. exilis; Madhok and Walker 1969). The first two mechanisms are likely not independent from each other (Kazakou et al. 2008). An example of plant adaptation from ultramafic areas to low content of Ca and high content of Mg is Achillea millefolium. O’Dell and Claassen (2006) collected seeds of this species from two various substrates (ultramafic soils, granites) and from a commercial source. Seeds were sown in pots with ultramafic soils. The accession of Achillea millefolium from granites and commercial source had necrosis in the root system when exposed to high Mg and low Ca contents of ultramafic soils. The accession from ultramafic areas had healthy roots.
Another factor causing serpentine syndrome is the low content of N, P, and K. Nagy and Proctor (1997) pointed out that plant growth in ultramafic areas at Meikle Kilrannoch (Scotland) is limited rather by the low content of P than N or K without affecting the phytoavailability of Ni and Mg. On the other hand, O’Dell et al. (2006) showed that vegetation in California (USA) is limited rather by N than by P. Moreover, in the study of Achillea millefolium, it was demonstrated that adverse conditions of ultramafic soils can be ameliorated by adding compost, and before compost decomposition, by fertilizer containing N, P, and K (O’Dell and Claassen 2006).
The growth of plants in ultramafic areas is also controlled by metal phytoavailability (Ni, Cr, Co, Mn). Nickel is the metal that is the most studied in plants because of its relatively higher phytoavailability compared to Cr and Co. Nickel is required in relatively low quantities in plants i.e. for urea metabolism (Uren 1992; Yusuf et al. 2011). However, elevated contents of Ni (> 10 mg kg−1) can cause nutrient imbalance, disruption of cell membrane functions, chlorosis, and necrosis (Yadav 2010; Yusuf et al. 2011). Plants colonizing ultramafic soils or other metalliferous soils display three strategies of metal transport: (a) exclusion, (b) indication and (c) accumulation (Baker 1981; Kazakou et al. 2008). To determine which strategy is used by a plant species, translocation factor (TF, see below) is helpful. The TF indicates the ability to translocate metals from roots to aboveground parts. Currently, research aiming plant communities in ultramafic areas are focused mostly on Ni-hyperaccumulators referred as species with Ni content of at least 1000 mg kg−1 in the dry matter of leaves i.e. Pycnandra acuminata (Reeves 1992; Reeves and Baker 2000; Reeves et al. 2007a; Van der Ent et al. 2013a; Gałuszka et al. 2015; Van der Ent et al. 2015). Furthermore, some Ni-hyperaccumulators give opportunity in phytomining operations because of high content of Ni and relatively high biomass (Nkrumah et al. 2016). The process involves cultivation of Ni-hyperaccumulators in large ultramafic massifs or contaminated areas. Afterwards, plants are harvested, combusted, thus giving “bio-ore”, and Ni is recovered as Ni salts or metallic Ni. Chaney et al. (2007) suggested that costs of phytomining are similar to the costs of food crops production, thus it gives economic opportunities for developing countries (i.e. Indonesia, Van der Ent et al. 2013b).
Very recently, several studies dealing with Ni isotopes composition of ultramafic rocks, soils derived from them and plants have been published (Ratié et al. 2015; Estrade et al. 2015; Ratié et al. 2016). The range of δ60Ni in ultramafic soils in Brazil (Barro Alto and Niquelândia) is from – 0.30 to 0.11 ‰ and in Albania (Qaftë Shtamë, Gjegjan, Prrenjas, Pojskë) from – 0.33 to 0.38 ‰ (Estrade et al. 2015; Ratié et al. 2016). Soils derived from ultramafic rocks are depleted in heavier Ni isotopes compared to the parent rock (Δ60Nisoil-rock was - 0.47 ‰ in Ratié et al. 2015 and Δ60Nisoil-rock was up to – 0.63 ‰ in Estrade et al. 2015). Authors suggested that secondary minerals (clay minerals and Fe-oxides) were responsible for the depletion in heavier isotopes. Fractionation of Ni in soils containing Fe-oxides as a main secondary mineral was higher (Δ60Ni soil-rock was - 0.60 ‰) than in soils containing smectite as a main secondary mineral (Δ60Ni soil-rock was - 0.20 ‰; Estrade et al. 2015). This was supported by isotope analysis of both exchangeable and phytoavailable pools that show heavier Ni signature than the solid. Nickel contained in whole plants growing in Albanian ultramafic area was isotopically heavier than that in the soil (Δ60Ni wholeplant-soil up to 0.40%; Estrade et al. 2015). Furthermore, non-hyperaccumulators translocated light Ni isotopes from roots to leaves (Δ60Ni leaves-roots up to - 0.60 ‰). In the case of hyperaccumulators, fractionation was observed only during early growth stage (Estrade et al. 2015). In other studies, Deng et al. (2014) cultivated the Ni - hyperaccumulator (Alyssum murale), the Ni, Zn - hyperaccumulator (Noccaea caerulescens) and a non - accumulator (Noccaea arvense) in hydroponic culture with low and high concentrations of metals in order to determine fractionation during accumulation. The isotopically light Ni was generally taken up by plants due to low-affinity transport system in cell membrane. However, the magnitude of fractionation was higher in hyperaccumulators (Δ60Ni plant-solution from - 0.63 ‰ to - 0.90 ‰) than non-hyperaccumulators (Δ60Ni plant-solution was - 0.21 ‰) growing in hydroponic culture.
The aim of the present paper is to determine how different types of parent ultramafic rocks, which results from their metamorphic transformations, affect the metal phytoavailability and the metal content in plants under temperate climate. To achieve this objective, we compared results of the DTPA-CaCl2 extraction with Ni isotopes of parent rocks and soils. Furthermore, we analyzed chemical composition of plants.
Materials and methods
Outline of climate, soils, and vegetation in lower Silesia
The climate of Lower Silesia belongs to temperate climates with the characteristics of a oceanic and continental climate (Głowicki et al. 2005 and references therein). Occasionally, the arctic and tropical air arrives to the studied area. Lower Silesia is characterized by a wide range of altitudes (from 70 m a. s. l. to 1603 m a. s. l.) with high diversified topography thus meteorological elements change rapidly in a small space. Long-term temperature and rainfall are not known precisely for study areas because of lack of the meteorological stations.
Distribution of soil cover in Lower Silesia is strongly influenced by variability of geological bedrock. Moreover, the whole region was overprinted in the Pleistocene with glacial sediments, therefore loess material, tills, and glaciofluvial sands significantly control soil evolution and classification (Kabała et al. 2015). Approximately 35% of the total region area is represented by Luvisols/Planosols derived from (1) aeolian silt or (2) stratified materials, layer of sand over loam (Musztyfaga and Kabała 2015; Kabała et al. 2015). Cambisols cover approximately 18% of Lower Silesia, Fluvisols/Fluvic Cambisols near 13%, Brunic Arenosols contribute with around 12%, whereas Chernozems, Mollic Gleysols, and Gleyic Pheozems cover 8% (Łabaz and Kabała 2014; Kabała et al. 2015). Podzols are less widespread (4%), their presence is limited to mountain regions (Waroszewski et al. 2016) and large sand plains. Overall, soils derived from ultramafic rocks in Poland are classified as Leptosols and Cambisols (Weber 1980). Parent rocks are represented by serpentinites with different stages of metamorphism. Ultramafic soils in Poland are generally shallow (up to 50 cm) with high amounts of rock fragments, which increase downwards the profiles. Kierczak et al. (2007) showed that Ni is less mobile in Polish ultramafic soils compared to other soils in Europe because climate in Poland represents colder and dryer variety of a temperate climate. Furthermore, in Polish soils, olivine is still observed even in surface horizons due to specific climate conditions and previous hydrothermal alteration of parent rocks.
Lower Silesia is characterized by highly diversified vegetation. The flora contains 1890 species which represent 76% of the whole Polish flora (Kącki et al. 2005 and references therein). Almost 34% of plants in Lower Silesia are threatened or rare. Postglacial characteristics of the flora and low isolation of the region are responsible for a small number of endemites emphasizing uniqueness of vegetation. The endemites mostly grow in mountainous part of Lower Silesia, i.e. Pedicularis sudetica. Plant communities are represented by forests both in mountains and in lowlands (i.e. Tilio platyphhyllis-Acerion pseudoplatani). Furthermore, meadow communities (i.e. Calthion palustris) are refuge for rare and protected species. Xerothermic, psammophilous and on the rock grasslands (i.e. Festucetalia valesiacae) occupy small areas (Kącki et al. 2005 and references therein). Moreover, 13 species in Lower Silesia are protected according to habitat directive of the European Union. One of the species listed in the habitat directive (Asplenium adulterinum) grows only in gaps of ultramafic rocks cropping out i.e. on Żmijowiec (Pędziwiatr 2015). The chemical composition of plants occurring in ultramafic areas in Poland was the subject of several studies (Brej and Fabiszewski 2006; Kasowska 2007; Żołnierz 2007; Samecka-Cymerman et al. 2008; Kubicka et al. 2015). The metallophytic flora is represented by Asplenium onopteris var. silesiaca and Noccaea caerulescens (Brej and Fabiszewski 2006). The highest content of Ni was found in Noccaea caerulescens from Mikołajów (3100 mg kg−1) while metal contents in other species were much lower. For example, the Ni, Cr, and Co contents in Asplenium onopteris var. silesiaca were 101 mg kg−1, 61 mg kg−1, and 21 mg kg−1 respectively; Brej and Fabiszewski 2006).
Study area and soil characteristics
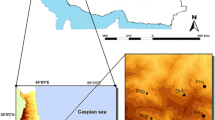
Studied materials (parent rocks, soils, and plants) were collected from two ultramafic sites previously described by Kierczak et al. (2016). We chose the Szklary Massif (site 1) and Jordanów (site 2) located in Lower Silesia (southwestern Poland; Fig. 1) because of the same position in the geomorphological sequence but differing in terms of parent rocks.
The Szklary Massif is located in the Fore Sudetic Block (Fig. 1). Soil from this locality is derived from partially serpentinized peridotite under lower range of greenschist facies (serpentine group minerals; olivine; amphibole; magnetite, Cr-magnetite, chlorite and orthopyroxene) and there is classified as Eutric Leptosol (Humic, Magnesic; Fig. 2; Gunia 2000; Kierczak et al. 2016). From a mineralogical point of view, soil contains (a) minerals inherited from parent rock or derived from weathering (serpentine, olivine, chlorite, talc) and (b) quartz as an allogenic mineral. Clay minerals are represented by smectite.
Jordanów (site 2) is located also in the Fore Sudetic Block (Fig. 1). The parent rock is represented by serpentinite (>90 vol.% of serpentine group minerals; Kierczak et al. 2016). Soil from site 2 correspond to Eutric Skeletic Cambisol (Loamic), Fig. 2, with serpentine and quartz in mineral composition. Swelling phases are represented by smectite similarly to site 1. Details of chemical composition of parent rocks and soils are presented in Table 1. In both studied sites, the SiO2 contents increase upward the profile (up to 58% in ABw horizon in site 2), whereas MgO contents increase downwards the profiles (up to 27% in BwC horizon in site 2). The highest contents of Ni are noted in site 1 in ABw horizon (2341 mg kg−1), whereas Cr contents in surface horizon (3503 mg kg−1). In site 2, the highest contents of Ni and Cr are noted in horizon above parent rock. The Co contents do not exceed 170 mg kg−1 in both sites.
Plant sampling and analysis
Plants from 56 species belonging to 22 families have been collected. Names of species are given after Flora Europaea (Tutin et al. 1968; Tutin et al. 1972; Tutin et al. 1976; Tutin et al. 1980; Tutin et al. 1993) and phytosociological affiliation is based on Mucina (1997). At least three specimens of single species of plants were collected in order to obtain an averaged sample and sufficient biomass for analysis. Plants were collected randomly in the distance of several meters from the soil profiles during efflorescence in 2014 and 2015. Underground and aboveground parts of plants were analyzed separately. Firstly, samples were rinsed in fresh water and next washed in distilled water avoiding contamination by soil and dust particles. Subsequently, samples were air dried and milled. Aliquot of the sample (1 g) was digested in concentrated HNO3 and then in aqua regia. Plants were analyzed by means of ICP-MS PerkinElmer NexION300 in Bureau Veritas Mineral Laboratories (Canada).
Chemical extraction
The DTPA-CaCl2 extraction was applied in assessment of phytoavailability of Ni, as it was used extensively for estimating the worst scenario of phytoavailable pool of metals (Lombini et al. 1998; Echevarria et al. 2006). The extraction was made in 0.005 DTPA and 0.01 M CaCl2 solution at pH = 5.3 in 1: 5 soil or pulverized rock to solution ratio (Quantin et al. 2008). The mixture was shaken for 2 h. Afterwards, suspension was centrifuged and filtered by 0.45 μm syringe filters (cellulose acetate membrane, VWR International®). All samples were prepared in duplicates with reagent blank samples. Nickel concentrations were measured by Flame Atomic Absorption Spectroscopy – VARIAN AA240FS at GEOPS laboratory. Phytoavailability of Ni extracted with the DTPA-CaCl2 solution was calculated as phytoavailable fraction relative to total content in soil or parent rock.
Isotope analysis
Preparation of samples for Ni isotope analyses consisted in digestion of rocks and soils followed by purification using two types of resins. Approximately 100 mg of crushed and homogenized sample was transferred to Teflon® beakers and digested with 5 mL of concentrated HF and 1.5 mL HClO4. After digestion, solutions were evaporated to dryness. In the next step mixture of concentrated HCl (3.75 mL) and HNO3 (1.25 mL) was added to residuum and evaporated. Afterwards, samples were treated with 3 mL of HNO3 and evaporated. This step was repeated. The residuum was taken with 2 mL of HNO3 and transferred to volumetric flasks and diluted with Mili-Q® water to 50 mL.
The chemical separation of Ni was performed according to protocol adopted by Gueguen et al. (2013). The aliquot of solutions (digested rocks and soils) and the DTPA-CaCl2 leachates were evaporated to dryness and conditioned in 6 M HCl. The Ni chemical purification of samples was based on a two-step chromatography separation. A first set ion-exchange chromatography columns was filled with 2 mL (wet volume) of anionic resin AG1-X8 in 6 M HCl (BioRad® 100–200 mesh). This resin retained Fe, Zn, and a high amount of Co and Cu (Moynier et al. 2007), while Ni remained in solution. Before the second chromatography column, a Ni double spike was added to the samples with a spike/natural ratio of 1 (Gueguen et al. 2013). The second set of ion-exchange chromatography columns use a specific Ni-resin (O.5 mL, wet volume) composed of polymethacrylate containing a dimethylglyoxime (DMG) molecule that retained Ni onto the resin as an insoluble Ni-DMG complex at pH 8–9, while the other elements were removed in solution. The eluted Ni solution was evaporated and taken up in 0.28 M HNO3 for measurement. Ni purification was performed at GEOPS laboratory and the Ni isotope ratios were measured with a Neptune (Thermo-electron) MC-ICP-MS at the Pôle Spectrométrie Océan (PSO), of IFREMER (Centre de Brest, France). The samples and standards were introduced via an ApexQ (50–75 V per μg/mL). A single “run” consisted of one block of 40 measurements. During the measurement, the Ni concentration (spike + natural) in the sample is 400 μg/L. The double spike is a mixture of 61Ni and 62Ni with a ratio of 1.1004. Application of a three-dimensional data reduction procedure was used to determine the true isotope ratios of the samples (Siebert et al. 2001). In addition, each sample analysis was bracketed by the measurements of the spiked standard Ni NIST SRM 986 solutions at the same concentration and same spike/standard ratio as the samples. The ratios of δ60Ni were expressed in per mil and normalized with the average value of the bracketing standard SRM-986 (Eq. 1; Gramlich et al. 1989).
The long-term analytical reproducibility (2SD) of the standard Ni NIST SRM 986 was typically comprised between 0.03 and 0.05 ‰.
The magnitude of fractionation between soils and rocks is estimated as Δ 60Nisoil-rock = δ60Nisoil - δ60Nirock and between phytoavailable fraction (DTPA-CaCl2) and soils is estimated based on Δ60NiDTPA/CaCl2-soil = δ60NiDTPA-CaCl2 - δ60Nisoil.
Translocation factor
The Translocation Factor (TF) is a widely used parameter showing ability to transferring metals from roots to aboveground parts of plants. The TF was calculated using equation (Eq. 2) given below:
where Cshoot and Croot is content of metals (mg kg−1) in aboveground and underground parts respectively.
Quality control and statistical analysis
The analytical reproducibility (2σ/2SD), as estimated from replicate analyses of composite samples Euphorbia cyparissias (aboveground part), Hieracium pilosella (aboveground part), Dianthus caarthusianorum (underground part), Achillea pannonica (underground part), Helianthemum nummularium (underground part) and Lepidium campestre (underground part) ranges from 0% (Ca) to 20% (Cr) at 95% confidence limits. Analytical accuracy (2σ), as estimated from 6 measurements of standard CDV-1 is from 0.2% (Ca) to 5% (Ni) at 95% confidence limits. Furthermore, analytical accuracy (2σ), as estimated from 7 measurements of standard V16 is from 1.4% (Cr) to 6.7% (Ca).
We compare chemical composition of plant populations from the serpentinized peridotite (site 1) and from the serpentinite (site 2) using the U-Mann Whitney test and the Cochran-Cox test. The normality of results was verified by the Kolmogorov – Smirnov test with Lillefors correction and homogeneity of variance by the Levene and Brown-Forsythe test. Effect size for the U-Mann Whitney test was calculated after Fritz et al. (2012). Relationships between elements in plants were analyzed by rank Spearman correlation (data with non-normal distribution) and Pearson linear correlation (data with normal distribution). Significance level (α) in this study is 0.05. For statistical analysis Statistica ver. 10. (StatSoft 2011) was applied. Results of statistical analysis are presented in supplementary materials (Online Resource 3–4) and selected results in appropriate figures.
Results
Chemical extraction
The chemical extraction of soils with the DTPA-CaCl2 demonstrates that Ni is more phytoavailable in soil derived from the serpentinized peridotite (site 1) compared with soil derived from the serpentinite (site 2; Fig. 3; Online Resource 1). The similarity of studied sites lies in the highest phytoavailability of Ni in surface horizons. In detail, the quantity and proportion of phytoavailable Ni in surface horizon in site is 138 mg kg−1 (6.5%), whereas in site 2 is 33 mg kg −1 (4%).
Vegetation
Plant species from Leguminosae, Graminae, and Compositae families have been most frequently collected in site 1 (8, 6 and 5 species respectively) and in site 2 from Compositae and Graminae (6 and 5 species respectively). However, it is not whole floristic compositions because not all species are analyzed and plants were collected randomly in this study. Details of floristic composition and community structure of vegetation occurring on Polish ultramafic soils are presented in the study of Żołnierz (2007). Nevertheless, it is worth to note that according to phytosociological affiliation of plants, the largest number of collected species (10 from site 1 and 15 species from site 2) is associated with the Festuco-Brometea class (xerothermic grassland; Online Resource 2) and is divided in Poland in two orders: Brometalia erecti and Festucetalia valesiacae (Szczęśniak 2003). In general, xerothermic grasslands are highly insolated and rich floristically. The phytosociological affiliation of Polish xerothermic grasslands developed on ultramafic rocks is discussed. According to Szczęśniak (2003), xerothermic grasslands in ultramafic areas belong to Brometalia erecti. However, in other studies, there are classified to Festucetalia valesiacae (Matuszkiewicz 2012). Furthermore, we observe that two grasses (Calamagrostis epigejos and Arrhenatherum elatius) compete for space with species representing Festuco-Brometea class (Fig. 2). Żołnierz (2007) called areas with these grasses as degraded xerothermic grasslands. It is noteworthy that among studied plants, Avenula pratensis, Achillea pannonica, and Salvia pratensis are listed on the local Red List (Lower Silesia; Kącki et al. 2003).
The chemical composition of plants is presented in Tables 2 and 3. In general, most of species from both localities is characterized by Ca/Mg ratio higher than 1. For example, the highest Ca/Mg ratio in site 1 is noted in aboveground parts of Plantago media. However, some species maintain relatively low Ca/Mg ratio i.e. Calamagrostis epigejos from site 2 represents species with low Ca/Mg ratio which is 0.3. On the other hand, this plant is characterized by the highest contents of Ni, Cr, and Co in both sites. The roots of Calamagrostis epigejos from site 1 contain 642 mg kg−1 of Ni and from site 2 content of this element is two times lower. The Cr content is higher in roots of Calamagrostis epigejos from site 2 (165 mg kg−1) compared with site 1 (97 mg kg−1), whereas Co content is similar in both areas. In both localities, the Ni, Cr, and Co are absorbed in greater amounts in roots compared with aboveground parts, thus TF of almost all species is lower than 1. Nevertheless, several species translocate effectively metals from roots to aboveground parts (i.e. TFCo in Sedum maximum from Jordanów is 1.31). Furthermore, species belonging to Graminae (i.e. Avenula pratensis, Calamagrostis epigejos) stand out generally by the lowest ability to metals translocation.
Ni isotopic composition
The isotopic signature (δ60Ni) of the serpentinized peridotite from the Szklary Massif (site 1) is 0.13 ± 0.06 ‰ and in the serpentinite from Jordanów (site 2) is 0.20 ± 0.06 ‰ (Table 4). The soil horizons from site 1 are isotopically lighter relative to the parent material: δ60Ni of uppermost horizon (A) is – 0.09 ± 0.06 ‰ and in ABw horizon 0.07 ± 0.07 ‰. The range of δ60Ni values of soil from site 2 is larger, from 0.01 ± 0.06 ‰ (BwC horizon) to 0.32 ± 0.07 ‰ (ABw horizon). Furthermore, the DTPA-CaCl2 extractable Ni displays heavy isotope composition in all horizons in site 1, with a Δ60NiDTPA/CaCl2-soil of +0.63 to +0.74 ‰ (Table 4). For site 2, the Δ60NiDTPA/CaCl2-soil is more variable, ranging from −0.56 to +0.12 ‰.
Discussion
Phytoavailability of metals in soils and Ni isotopic fractionation
The results of the chemical extraction with the DTPA-CaCl2 show that type of ultramafic parent material appears to affect the Ni phytoavailability. Kierczak et al. (2016) have explained higher phytoavailability of Ni in soils derived from the partially serpentinized peridotite (the EDTA extraction) compared to the soils derived from the serpentinite due to fact that olivines (Ni-bearing phase in site 1) are more susceptible to weathering than serpentine group minerals (Ni-bearing phase in site 2). Furthermore, the authors have shown that the Ni phytoavailability is higher than Co and Cr. Phytoavailability of Cr has been low in soils due to fact that this element is bound in the spinel group of minerals characterized by low susceptibility to weathering. The phytoavailability of metals can be controlled also by organic matter. The soil from site 1 is characterized by higher content of organic carbon (up to 4% in A horizon) compared to the soil from site 2 (up to 2% in ABw horizon). The organic matter in the soil from site 1 is able to retain more Ni on exchangeable positions compared to site 2 thereby, higher phytoavailability of Ni is observed in site 1 than site 2 (Echevarria et al. 2006). The phytoavailability of metals can be regulated by clay minerals. In the study of Echevarria et al. (2006), the Ni was more phytoavailable in soils, which were richer in smectite, as a result of presence exchangeable positions similarly to the organic matter. The smectite is present in both studied soils but it is difficult to determine proportion of this mineral in soils based on XRD observations. Moreover, the phytoavailability of metals can be controlled by crystalline Fe-oxides that can be considered as stable sink of metals under oxic conditions. In contrast, the amorphous Fe-oxides are considered as available source of Ni because of the sorption of Ni on the surface rather than incorporation within crystal lattice observed in crystalline Fe-oxides (Massoura et al. 2006; Chardot et al. 2007). However, the Fe-oxides were not identified during XRD studies, even, if Kierczak et al. (2007) previously observed oxy-hydroxides - clayous mixture in soil from site 1 by SEM and EMPA.
The Ni isotopic composition of the partially serpentinized peridotite and serpentinite is close to the parent rocks presented in other studies (Fig. 4; Gueguen et al. 2013; Estrade et al. 2015). The slight differences in Ni isotopic composition of parent rocks from various ultramafic massifs can be explained by variabilities of parent magma and degree of serpentinization causing a possible isotopic fractionation (Ratié et al. 2015; Estrade et al. 2015). In the last studies, Gall et al. (2017) have suggested that serpentinization probably does not affect δ60Ni. Our results support this finding to some extent because δ60Ni for both parent rocks is in the range noted for unweathered peridotite xenoliths (from 0.09 ‰ to 0.32 ‰; Gall et al. 2017). Furthermore, the authors have explained differences in δ60Ni of ultramafic rocks by proportion of clinopyroxene that represents heavy Ni isotope composition (up to 2.83 ± 0.11 ‰) compared to olivine (up to 0.17 ± 0.05 ‰) or orthopyroxene (up to – 0.04 ± 0.04 ‰).
Isotopic composition of the Leptosol in site 1 shows that soil is lighter than parent material i.e. Δ60Nisoil-rock for A horizon is – 0.22 ‰. This trend is previously reported in Albania (Δ60Nisoil-rock is up to - 0.63 ‰) and at Barro Alto (Estrade et al. 2015; Ratié et al. 2015). The Ni isotope composition of phytoavailable fraction estimated using the DTPA-CaCl2 solution shows that heavier Ni isotope pool is released during weathering of ultramafic rocks. In detail, the Δ60NiDTPA/CaCl2-soil for A and ABw horizon is 0.74 ‰ and 0.63 ‰ respectively. The magnitude of fractionation (Δ60NiDTPA/CaCl2-soil) in Albanian’s ultramafic soils was up to 0.89 ‰. Our results for soil from site 1 support to some extent findings that pedogenesis leads to removal isotopically heavy phytoavailable pool of Ni.
The Ni isotope composition of the Cambisol from site 2 is more diverse. The Bw and BwC horizons are lighter than parent material confirming previous results. On the contrary, the surface horizon (ABw) has heavy isotopic composition. We suggest that decomposition of aboveground parts of plants can explain to some extent heavy isotope composition of soil in the surface horizon from this locality. Studies in ultramafic areas in Albania demonstrated that the litter was isotopically heavier than rhizospheric soil thus, decomposition of litter can deliver heavy isotopes to the surface horizon. Very recently, Šillerová et al. (2017) suggested that biological and biochemical fractionation is responsible for heavy isotope composition of topsoil from Norway (δ60Ni up to 1.71 ‰). Furthermore, experiments simulating litter decomposition on the Rinorea bengalensis (Ni-hyperaccumulator) showed that 80% of Ni was released during first 10 days of the experiment. The released pool of Ni was enriched in heavy isotopes (Δ 60Nileached10days-leached30days = 0.20 ‰; Zelano et al. 2017).
The phytoavailable fraction from Bw horizon of the Cambisol in site 2 presents only heavy isotope composition. It is difficult to clearly explain light isotopic composition of phytoavailable fraction in ABw and BwC horizons. Estrade et al. (2015) pointed out that differences in isotope composition between various soils (Cambisol and Vertisol) can be explained by functions and pedogenesis of these soils. We study the Leptosol (site 1) and the Cambisol (site 2) thus, differences between these soils can result from pedogenesis. The slight distinction between these soils is reflected in the spatial distribution of the elements. The Ni content in soil relative to the parent rock is rather stable in site 1 (Fig. 5). On the other hand, the enrichment of Ni is observed in BwC horizon and depletion in ABw horizon in site 2. Chromium is stable in both sites (except the surface horizon in site 1) and Co is enriched in site 1 compared to site 2. Calcium and Mg are similarly distributed in both soil profiles relative to the parent rocks. The relevant difference between studied soils is noticeable in normalization of the Ni content between soils and rocks to the immobile Al. The [Ni/Al]soil/[Ni/Al]rock demonstrates that weathering process is more advanced in site 2 than site 1 (Table 4; calculated after Estrade et al. 2015). The comparison of the [Ni/Al]soil/[Ni/Al]rock relative to the δ60Ni of soils shows surprisingly that the more advanced weathering, the heavier isotopic composition of soils (Fig. 5). It suggests that light pool of Ni could have leached out during pedogenesis in Polish soils. However, this is not reflected in the δ60Ni of the DTPA-CaCl2 fraction of the soils. The DTPA extraction of the soils is considered as efficient to assess phytoavailability of metals (Estrade et al. 2015 and references therein). On the other hand, it is possible that during 2 h of the extraction the entire pool of phytoavailable Ni is not extracted because of diffusion controlled processes that may affect Ni isotopic signature.
Accumulation of metals by plants
The ecological peculiarity of ultramafic areas is related to the excess of Mg2+ at deficiency of Ca2+ in soils (Proctor 1970; Becquer et al. 2010). The imbalance between these cations in soils (Ca/Mg ratio < 1) together with presence of metals are responsible for toxicity for plants. Our results support hypothesis that imbalance between exchangeable Ca and Mg in soils is an important factor controlling vegetation because Ca/Mg ratio in most horizons is lower than one (from 0.27 to 0.77) except for the surface horizon in site 2 (1.19). Furthermore, metal contents in soils are in the range published in other studies.
The results of chemical composition of studied plants are generally consistent with previous results for plants from grasslands developed on ultramafic rocks and spontaneous vegetation on waste dumps in Poland (Żołnierz 2007; Koszelnik-Leszek and Kasowska 2009). All species, both in above- underground parts are characterized by the highest contents of Ni followed by Cr and Co. The same tendency was observed in other studies independently the climate conditions (Kataeva et al. 2004; Reeves et al. 2007a; Reeves et al. 2007b; Lago-Vila et al. 2015). For example, leaves of Bonamia mexicana from Santa Elena peninsula (Costa Rica) accumulated 12 mg kg−1 of Ni followed by 0.5 mg kg−1 of Cr and 0.1 mg kg−1 of Co. Most of the species is characterized by the TF for Ni, Cr, and Co lower than 1 suggesting strategy for exclusion in Polish ultramafic vegetation. It was reported also for plants from Spain, USA, and Taiwan (Oze et al. 2008; Lago-Vila et al. 2015; Gonneau et al. 2017; Hseu and Lai 2017). The exclusion can be explain by sequestration of metals in the vacuoles of roots or binding to the exchange sites of cell walls in xylem (Seregin and Kozhevnikova 2006; Yusuf et al. 2011).
Taking into account higher phytoavailability of metals in soils from site 1 compared to site 2 we expected similarly higher contents of these elements in plants from site 1. However, we observed another tendency especially with regard to aboveground parts (Online Resource 3). Aboveground parts of plants from site 2 have significantly higher content of Ni (i.e. MdnA = 24.35 mg kg−1) than plants from site 1 (i.e. MdnA = 13.40 mg kg−1; Z = − 3.88, p < 0.05, r = − 0.45). Studied species from site 2 have also significantly higher content of Cr both in aboveground parts (Z = − 3.31, p < 0.05, r = − 0.39) and underground parts (Z = − 3.69, p < 0.05, r = − 0.43). For Co difference is noted only in underground parts (Z = − 1.98, p < 0.05, r = − 0.23). On the contrary, aboveground parts of plants from site 1 are characterized by higher Ca/Mg ratio than plants from site 2 (t = 3.22, p < 0.05; Online Resource 3). The results of statistical analysis can partly indicate alleviating role of Ca and/or Mg in the case of high content of metals in soils. One way to confirm this hypothesis is to look at the difference in physicochemical composition of studied soils. In detail, the Leptosol from site 1 derived from the serpentinized peridotite has higher contents of exchangeable Ca (up to 20.8 cmol (+) kg−1 in A horizon) and Mg (up to 59.9 cmol (+) kg−1 in ABw horizon) than the Cambisol from site 2 derived from the serpentinite (exchangeable Ca and Mg do not exceed 7.0 cmol (+) kg−1 and 9.1 cmol (+) kg−1 respectively in BwC horizon). Therefore, it is possible that exchangeable Ca can compete with metals in plants from site 1. The competition between Ca and metals was observed by other authors (Li et al. 2009; Wang et al. 2010; Yusuf et al. 2011; Lago-Vila et al. 2015; Aziz et al. 2015). For example, Li et al. (2009) and Lago-Vila et al. (2015) suggested that exchangeable Ca can decrease Ni and Co accumulation by plants because of competition for binding sites in root cells. Furthermore, Wang et al. (2010) studied relationships between Ca2+ and Cu2+, Cd2+, Ni2+ in Triticum aestivum and Pisum sativum. In detail, after Ca2+ was added to the growth medium, electric potential in the outer surface of cell membrane decreased. Consequently, activity of metals was lower and toxicity was alleviated. Aziz et al. (2015) used rice for studying interactions between Ca and Ni. The presence of Ca in tested soils caused decrease in Ni content in plants due to improvement metabolic functions of cell membrane and maintaining its integrity. Furthermore, the Ca caused increase in chlorophyll content and increase in the rate of transpiration.
High contents of Mg in ultramafic soils are known as toxic for plants (Proctor 1970). Most of the plants from both localities have Ca/Mg ratio higher than 1 suggesting that plants overcome high content of Mg and low of Ca in soils due to adaptation mechanisms. This is in agreement with other studies (Pandolfini and Pancaro 1992; Lombini et al. 1998; Oze et al. 2008). Despite the toxic role of Mg for plants, it is possible that this element is also able for limiting accumulation of metals. Studies of surface potential of plasma membrane in plants (not from ultramafic areas) revealed that Mg can compete with metals because of decrease negativity of the plasma membrane surface electrical potential (Kinraide 2006). Furthermore, important insight into the alleviating role of Ca and Mg comes from studies of ion channels. For instance, the Ca2+ channel “rca” is able to transport monovalent (i.e. Na+) and divalent cations (i.e. Ni2+, Cu2+, Mg2+) if there is no Ca2+ (White 2000). It suggests that competition between Ca2+, Mg2+, and Ni2+ seems possible. Studies with Berkheya coddii demonstrated also that Ni accumulation can be inhibited by both Ca and Mg (Robinson et al. 1999). Correlation analysis of species in our study supports alleviating role of Ca2+ and Mg2+ (Fig. 6; Online Resource 4). For instance, rank Spearman correlation coefficient between Ni and Mg of aboveground parts in site 1 is 0.65 (p < 0.05). Similarly to our results, Ater et al. (2000) found significant positive correlation between Mg and Ni in plants from North Morocco suggesting that plants growing on ultramafic soils cope with high content of Ni and Mg in soils by the same mechanisms (exclusion and accumulation). Similar observations were also reported in the study of plants growing in Europe (Shewry and Peterson 1971).
The differences in chemical composition of plants indicate that type of ultramafic parent material affects metals accumulation by plants. The main Mg-bearing phase in the serpentinized peridotite is olivine and serpentine and only serpentine in the serpentinite. We observe higher content of exchangeable Mg in soils derived from the serpentinized peridotite because olivine is more extensively weathered than serpentine. Higher content of exchangeable Ca in site 1 is related to the presence of amphibole (tremolite – a Ca bearing mineral, Kierczak et al. 2007) which does not occur in site 2.
Another explanation of differences in the accumulation of Ni, Cr, and Co in studied plants can be attributed to changes in pH. Mobility of metals increases when pH of soil decreases (Greger 1999). Soil from site 2 has slightly lower pH (6.3 in uppermost horizon) compared to soil from site 1 (pH = 7 in uppermost horizon; Kierczak et al. 2016). Lower pH of soil from site 2 could promote Ni, Cr, and Co accumulation by plants from this locality.
Broadhurst et al. (2009) studied interactions between Mn and Ni in two hyperaccumulators (Alyssum murale and Alyssum corsicum). Authors observed that phytoextraction of Ni by Alyssum species can be reduced by more available Mn. Our results showed that soil from site 1 contain higher content of Mn (up to 1781 mg kg−1 in A horizon) compared to soil from site 2 (up to 774 mg kg−1 in ABw horizon; Kierczak et al. 2016). Higher content of Mn in ultramafic soils from site 1 and significant correlation between Ni, Cr, Co, and Mn in plants support hypothesis that Mn reduces metal accumulation by plants (Fig. 7; Online Resource 4). Furthermore, these results demonstrate that type of ultramafic rocks affects metal accumulation by plants. Olivine and pyroxene in the serpentinized peridotite from site 1 are the most enriched in Mn (average 929 mg kg−1 and 1936 mg kg−1 respectively; Kierczak et al. 2016), whereas serpentine in the serpentinite from site 2 contain with an average of 542 mg kg−1 of Mn. Olivines are easily weatherable thus they could release Mn which possibly reduces accumulation of other metals by plants. Similarly to olivines, pyroxenes weather to hydrous layer silicates (Wilson 2004) supplying soils also in Mn. The significant positive correlation between Cr and Mn in plants was found also in the study of Megremi (2010) suggesting strong relationships between both elements. The U-Mann Whitney test revealed surprisingly significant higher content of Mn in plants from site 2 than site 1 although the fact that soil from site 1 contains higher content of Mn. Soil from site 2 is characterized by higher content of clay fraction compared to site 1 hence possibly less drained. Therefore, higher moister of soils in site 2 can induce reduction of Mn oxides and release available Mn.
We found also positive significant correlation between Ni, Cr, and Co in plants regardless of the study area (Fig. 6; Online Resource 4). Rencz and Shilts (1990) suggested that correlations between these elements reveal strong interrelationships between metals. Moreover, Megremi (2010) found a significant positive correlation between Ni and Cr, similarly to our study.
The complexity in the study of plants from ultramafic soils
Species growing in ultramafic soils are extensively studied for many years (i.e. Whittaker 1954; Main 1974; Main 1981; Galey et al. 2017; Gonneau et al. 2017; Van der Ent et al. 2017; Van der Ent et al. 2018). Although knowledge about relationships between metals with other elements or factors in plants is broad, it is still difficult to final identify the causes of differences in Ni, Cr, and Co content in plants from Polish ultramafic soils. Some authors suggested that accumulation of metals depends on the nature of plants (i.e. differences in genetic composition between species or specimens; Greger 1999). Another factor affecting accumulation of metals can be attributed to anthropogenic pollution. According to Becquer et al. (1992), acid rain (containing NO3 − and SO4 2−) modifies pH of soils and Al toxicity. It was observed that during vegetation season coniferous trees from Vosges Mountains (France) are able to take up NO3 − ions. At the same time, Ca2+, Mg2+, and K+ are extracted from plants by acid rains leading to alkalinization and decreasing Al toxicity. The Szklary Massif and Jordanów are covered mainly by xerothermic grassland. However, it is possible that NO3 − in rain can affect the pH of soils and metals contents in plants.
The variation in the chemical composition of plants can be explained also by presence of glacial material in ultramafic soils. Rencz and Shilts (1990) consider that glaciation affects species composition, vigor of plant communities, and change of the cation exchange capacity in the soil-roots system. Glacial material could deliver additional amount of Si which is considered as an element alleviating toxicity of metals when is absorbed by plants (Adrees et al. 2015).
Our field observations and results of chemical composition of plants do not show presence of endemites and Ni-hyeraccumulators. In our opinion, presence of glacial material in Polish soils can be responsible for the lack of these species in studied communities. Furthermore, it was also demonstrated that age of ultramafic area affects the number of endemites (reviewed in Proctor and Nagy 1991) thus relatively young age of Polish ultramafic soils can be responsible for lack of these species. On the other hand, plant growth in ultramafic soils can lead to the formation ecotypes and afterwards endemites (Anacker 2014), thus it is possible that Polish flora in ultramafic areas move towards endemism.
Conclusions
The chemistry of soils and plants is controlled by the mineral composition of parent rocks, which results from both their original magmatic composition and later metamorphic processes. Soil derived from the serpentinized peridotite (Leptosol) is shallower compared to soil formed from the serpentinite (Cambisol). Chemical extractions show that the phytoavailability of Ni and Co is higher in soil developed from the serpentinized peridotite than the serpentinite. Surprisingly, plants growing on the soils derived from the serpentinite contain higher levels of metals compared to these from the serpentinized peridotite derived soils. This contrasting behavior is due to higher abundances of Ca and Mg, not only Ni and Co, in soils developed from the serpentinized peridotite as compared to those in the soils derived from the serpentinite. Calcium and Mg are favored by plants and preferably fill the available sites, resulting in low Ni and Co intake despite their higher abundances. Differences between studied soils are also reflected in the isotopic composition. The isotopic signature of surface horizon in the Leptosol is lighter relative to the parent rock. On the other hand, enrichment in heavy isotopes is observed in surface horizon in the Cambisol. Furthermore, the phytoavailable pool of Ni extracted with the DTPA-CaCl2 in the Leptosol presents heavy isotopic signature, whereas in the Cambisol only in the middle part of the profile. Our results are only to some extent consistent with observations in soils derived from peridotites and serpentinites (Alexander 2009; Alexander and DuShey 2011). Soil derived from the serpentinized peridotite is not redder compared to soil developed from the serpentinite. The topographic differences are not visible because both sites are characterized by similar topography. However, our study is in agreement with observations that vegetation cover on soils derived from both types of rocks is generally similar. These observations are characteristics for temperate climate. The high degree of endemism sensu Whittaker (1954) is not visible in Polish ultramafic sites. In tropical zone, the vegetation growing in serpentinite derived soils (Cambisols, Leptosols) is characterized by high level of endemism (Van der Ent et al. 2018). Soils developed on peridotites (Ferralsols) are covered by rainforests, not so different from that on non-ultramafic soils. Furthermore, the vegetation cover in the Szklary Massif and Jordanów distinguishes from neighboring areas.
Abbreviations
- MdnA :
-
Median of aboveground parts
- MdnU :
-
Median of underground parts
- α:
-
Significance level
- p:
-
Probability level
- r:
-
Effect size for U-Mann Whitney test
- rs :
-
Rank Spearman correlation coefficient
- TF:
-
Translocation factor
References
Adrees M, Ali S, Rizwan M, Zia-ur-Rehman M, Ibrahim M, Abbas F, Farid M, Qayyum MF, Irshad MK (2015) Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: a review. Ecotoxicol Environ Saf 119:186–197. https://doi.org/10.1016/j.ecoenv.2015.05.011
Alexander EB (2009) Soil and vegetation differences from peridotite to Serpentinite. Northeast Nat 16(5):178–192. https://doi.org/10.1656/045.016.0515
Alexander EB, DuShey J (2011) Topographic and soil differences from peridotite to serpentinite. Geomorphology 135:271–276. https://doi.org/10.1016/j.geomorph.2011.02.007
Anacker BL (2014) The nature of serpentine endemism. Am J Bot 101:219–224. https://doi.org/10.3732/ajb.1300349
Ater M, Lefèbvre C, Gruber W, Meerts P (2000) A phytogeochemical survey of the flora of ultramafic and adjacent normal soils in North Morocco. Plant Soil 218:127–135
Aziz H, Sabir M, Ahmad HR, Aziz T, Zia-ur-Rehman M, Hakeem KR, Ozturk M (2015) Alleviating effect of calcium on nickel toxicity in Rice. CLEAN - Soil, Air, Water 43:901–909. https://doi.org/10.1002/clen.201400085
Badura J, Dziemiańczuk E (1981) The geological map of the Sudety Mountains - Ząbkowice Śląskie. Geological Institute, UK
Baker AJM (1981) Accumulators and excluders - strategies in the response of plants to heavy metals. J Plant Nutr 3:643–654
Becquer T, Boudot JP, Merlet D, Rouiller J (1992) Influence of N and S cycles on the proton balance in a declining fir forest. Relation with aluminium toxicity Comptes Rendus l’Académie des Sci -Pedologie 314:527–532
Becquer T, Quantin C, Boudot JP (2010) Toxic levels of metals in Ferralsols under natural vegetation and crops in New Caledonia. Eur J Soil Sci 61:994–1004. https://doi.org/10.1111/j.1365-2389.2010.01294.x
Brej T, Fabiszewski J (2006) Plants accumulating heavy metals in the Sudety Mts. Acta Soc Bot Pol 75:61–68
Broadhurst LC, Tappero RV, Maugel TK, Erbe EF, Sparks DL, Chaney RL (2009) Interaction of nickel and manganese in accumulation and localization in leaves of the Ni hyperaccumulators Alyssum Murale and Alyssum Corsicum. Plant Soil 314:35–48. https://doi.org/10.1007/s11104-008-9703-4
Chaney RL, Angle S, Li Y, Baker AJM (2007) Recovering metals from soils. US patent 7268273B2 (11 September 2007)
Chardot V, Echevarria G, Gury M, Massoura S, Morel JL (2007) Nickel bioavailability in an ultramafic toposequence in the Vosges Mountains (France). Plant Soil 293:7–21. https://doi.org/10.1007/s11104-007-9261-1
Coleman RG, Jove C (1992) Geological origin of serpentinites. In: Baker AJM, Proctor J, Reeves RD (eds) The vegetation of ultramafic (serpentine) soils. Intercept, Andover, pp 1–17
Deng THB, Cloquet C, Tang YT, Sterckeman T, Echevarria G, Estrade N, Morel JL, Qiu RL (2014) Nickel and zinc isotope fractionation in hyperaccumulating and nonaccumulating plants. Environ Sci Technol 48:11926–11933. https://doi.org/10.1021/es5020955
Echevarria G, Massoura ST, Sterckeman T, Becquer T, Schwartz C, Morel JL (2006) Assessment and control of the bioavailability of nickel in soils. Environ Toxicol Chem 25:643–651. https://doi.org/10.1897/05-051r.1
Estrade N, Cloquet C, Echevarria G, Sterckeman T, Deng T, Tang Y, Morel JL (2015) Weathering and vegetation controls on nickel isotope fractionation in surface ultramafic environments (Albania). Earth Planet Sci Lett 423:24–35. https://doi.org/10.1016/j.epsl.2015.04.018
Fritz CO, Morris PE, Richler JJ (2012) Effect size estimates : current use , calculations , and interpretation. Jour Exper Psycho 141:2–18. https://doi.org/10.1037/a0024338
Galey ML, Van der Ent A, Iqbal MCM, Rajakaruna N (2017) Ultramafic geoecology of south and Southeast Asia. Bot Stud 58:18. https://doi.org/10.1186/s40529-017-0167-9
Gall L, Williams HM, Siebert C, Halliday AN, Harrington RJ, Hein JR (2013) Nickel isotopic compositions of ferromanganese crusts and the constancy of deep ocean inputs and continental weathering effects over the Cenozoic. Earth Planet Sci Lett 375:148–155. https://doi.org/10.1016/j.epsl.2013.05.019
Gall L, Williams HM, Halliday AN, Kerr AC (2017) Nickel isotopic composition of the mantle. Geochim Cosmochim Acta 199:196–209. https://doi.org/10.1016/j.gca.2016.11.016
Gałuszka A, Krzciuk K, Migaszewski ZM (2015) A new two-step screening method for prospecting of trace element accumulating plants. Int J Environ Sci Technol 12:3071–3078. https://doi.org/10.1007/s13762-014-0719-4
Głowicki B, Otop I, Urban G, Tomczyński K (2005) Climate. In: Eco-physiographic description for Lower Silesia. Zarząd Województwa Dolnośląskiego, Wojewódzkie Biuro Urbanistyczne we Wrocławiu. in Polish, http://www.eko.wbu.wroc.pl/eko. Accessed 11 Dec 2016
Gonneau C, Mohanty SK, Dietterich LH, Hwang W, Willenbring JK, Casper BB (2017) Differential elemental uptake in three pseudo-metallophyte C 4 grasses in situ in the eastern USA. Plant Soil. https://doi.org/10.1007/s11104-017-3198-9
Gramlich JW, Machlan LA, Barnes IL, Paulsen PJ (1989) Absolute isotopic abundance ratios and atomic weight of a reference sample of nickel. J Res Natl Inst Stand Technol 94(6):347–356. https://doi.org/10.6028/jres.094.034
Greger M (1999) Metal availability and bioconcentration in plants. In: Prasad, Majeti, Narasimha V (ed) heavy metal stress in plants-from molecules to ecosystems. Springer-Verlag, Berlin, pp 1–27
Gueguen B, Rouxel O, Ponzevera E, Bekker A, Fouquet Y (2013) Nickel isotope variations in terrestrial silicate rocks and geological reference materials measured by MC-ICP-MS. Geostand Geoanalytical Res 37:297–317. https://doi.org/10.1111/j.1751-908X.2013.00209.x
Gunia P (2000) The petrology and geochemistry of mantle-derived basic and ultrabasic rocks from the Szklary massif in fore-Sudetic block (SW Poland). Geol Sudetica 33:71–83
Hseu Z-Y, Lai Y-J (2017) Nickel accumulation in paddy rice on serpentine soils containing high geogenic nickel contents in Taiwan. Environ Geochem Health. https://doi.org/10.1007/s10653-017-9925-6
Jenny H (1980) The soil resource: origin and behavior. Ecol Stud 37:256–259
Kabała C, Bekier J, Bińczycki T, Bogacz A, Bojko O, Cuske M, Ćwieląg-Piasecka I, Dębicka M, Gałka B, Gersztyn L, Glina B, Jamroz E, Jezierski P, Kabała C, Karczewska A, Kaszubkiewicz J, Kawałko D, Kierczak J, Kocowicz A, Krupski M, Kusza G, Łabaz B, Marzec M, Medyńska-Juraszek A, Musztyfaga E, Perlak Z, Pędziwiatr A, Pora E, Przybył A, Strączyńska S, Szopka K, Tyszka R, Waroszewski J, Weber J, Woźniczka P (2015) Soils of lower Silesia: origins, diversity and protection. Polish Society of Soil Science - Wrocław Branch, Polish HumicSubstances Society, Wrocław. in Polish
Kącki Z, Dajdok Z, Szczęśniak E (2003) The red list of vascular plants of Lower Silesia. In: Kącki Z (ed) Endangered vascular plants of Lower Silesia. Instytut Biologii Roślin Uniwersytetu Wrocławskiego, Polskie Towarzystwo Przyjaciół Przyrody“ Pro Natura,” Wrocław, pp 9–65. in Polish
Kącki Z, Szczęśniak E, Anioł-Kwiatkowska J (2005) Flora. In: Eco-physiographic description for Lower Silesia. Zarząd Województwa Dolnego Śląska, Wojewódzkie Biuro Urbanistyczne we Wrocławiu. in Polish, http://www.eko.wbu.wroc.pl/eko. Accessed 11 Dec 2016
Kasowska D (2007) Flora of xerothermic grasslands and ruderal communities of the Nasławice serpentine quarry (Kamienny Grzbiet ridge, Ślęża massif). Ann Silesiae 35:105–113 in Polish
Kataeva MN, Alexeeva-Popova NV, Drozdova IV, Beljaeva AI (2004) Chemical composition of soils and plant species in the polar Urals as influenced by rock type. Geoderma 122:257–268. https://doi.org/10.1016/j.geoderma.2004.01.012
Kazakou E, Dimitrakopoulos PG, Baker AJM, Reeves RD, Troumbis AY (2008) Hypotheses, mechanisms and trade-offs of tolerance and adaptation to serpentine soils: from species to ecosystem level. Biol Rev Camb Philos Soc 83:495–508. https://doi.org/10.1111/j.1469-185X.2008.00051.x
Kierczak J, Neel C, Bril H, Puziewicz J (2007) Effect of mineralogy and pedoclimatic variations on Ni and Cr distribution in serpentine soils under temperate climate. Geoderma 142:165–177. https://doi.org/10.1016/j.geoderma.2007.08.009
Kierczak J, Pędziwiatr A, Waroszewski J, Modelska M (2016) Mobility of Ni, Cr and co in serpentine soils derived on various ultrabasic bedrocks under temperate climate. Geoderma 268:78–91. https://doi.org/10.1016/j.geoderma.2016.01.025
Kinraide TB (2006) Plasma membrane surface potential (ψPM) as a determinant of ion bioavailability: a critical analysis of new and published toxicological studies and a simplified method for the computation of plant ψPM. Environ Toxicol Chem 25:3188–3198
Koszelnik-Leszek A, Kasowska D (2009) Characterization of vascular flora and accumulation of heavy metals in serpentine wastes and selected plant species from the excavation of the Nasławice quarry (lower Silesia). Ochr Środowiska i Zasobów Nat 41:640–650 in Polish
Kruckeberg AR (2004) Geology and plant life: the effects of landforms and rock types on plants. University of Washington Press, Seattle
Kubicka K, Samecka-Cymerman A, Kolon K, Kosiba P, Kempers AJ (2015) Chromium and nickel in Pteridium Aquilinum from environments with various levels of these metals. Environ Sci Pollut Res Int 22:527–534. https://doi.org/10.1007/s11356-014-3379-5
Łabaz B, Kabała C (2014) Origin, properties and classification of “black earths”. In: Poland. Soil Sci Annu 65, pp 80–90. https://doi.org/10.2478/ssa-2014-0012
Lago-Vila M, Arenas-Lago D, Rodríguez-Seijo A, Andrade Couce ML, Vega FA (2015) Cobalt, chromium and nickel contents in soils and plants from a serpentinite quarry. Solid Earth 6:323–335. https://doi.org/10.5194/se-6-323-2015
Li H-F, Gray C, Mico C, Zhao FJ, McGrath SP (2009) Phytotoxicity and bioavailability of cobalt to plants in a range of soils. Chemosphere 75:979–986. https://doi.org/10.1016/j.chemosphere.2008.12.068
Lombini A, Dinelli E, Ferrari C, Simoni A (1998) Plant – soil relationships in the serpentinite screes of Mt . Prinzera (Northern Apennines, Italy). J Geochemical Explor 64:19–33
Madhok OP, Walker RB (1969) Magnesium nutrition of two species of sunflower. Plant Physiol 44:1016–1022
Main JL (1974) Differential responses to magnesium and calcium by native populations of Agropyron Spicatum. Am J Bot 61:931–937
Main JL (1981) Magnesium and calcium nutrition of a serpentine endemic grass. Am Midl Nat 105:196–199
Marrs RH, Proctor J (1976) The responses of serpentine and non-serpentine Agrostis Stolonifera to magnesium and calcium. J Ecol:953–964
Massoura ST, Echevarria G, Becquer T, Ghanbaja J, Leclerc-Cessac MJL (2006) Control of nickel availability by nickel bearing minerals in natural and anthropogenic soils. Geoderma 136:28–37. https://doi.org/10.1016/j.geoderma.2006.01.008
Matuszkiewicz W (2012) Guide for the determination of plant communities in Poland. Wydawnictwo Naukowe PWN Sp. z o.o., Warszawa, in Polish
Megremi I (2010) Distribution and bioavailability of Cr in central Euboea, Greece. Open Geosci 2:103–123. https://doi.org/10.2478/v10085-009-0042-3
Moynier F, Blichert-Toft J, Telouk P, Luck JM, Albarede F (2007) Comparative stable isotope geochemistry of Ni , cu , Zn , and Fe in chondrites and iron meteorites. Geochim Cosmochim Acta 71:4365–4379. https://doi.org/10.1016/j.gca.2007.06.049
Mucina L (1997) Conspectus of classes of european vegetation. Folia Geobot Phytotaxon 32:117–172
Musztyfaga E, Kabała C (2015) Lithological discontinuity in Glossic Planosols (Albeluvisols) of lower Silesia (SW Poland). Soil Sci Annu 66:180–190. https://doi.org/10.1515/ssa-2015-0035
Nagy L, Proctor J (1997) Plant growth and reproduction on a toxic alpine ultramafic soil: adaptation to nutrient limitation. New Phytol 137:267–274. https://doi.org/10.1046/j.1469-8137.1997.00799.x
Nkrumah PN, Baker AJM, Chaney RL, Erskine PD, Echevarria G, Morel JL, van der Ent A (2016) Current status and challenges in developing nickel phytomining: an agronomic perspective. Plant Soil 406:55–69. https://doi.org/10.1007/s11104-016-2859-4
O’Dell RE, Claassen VP (2006) Serpentine and nonserpentine Achillea Millefolium accessions differ in serpentine substrate tolerance and response to organic and inorganic amendments. Plant Soil 279:253–269. https://doi.org/10.1007/s11104-005-2360-y
O’Dell RE, James JJ, Richards JH (2006) Congeneric serpentine and nonserpentine shrubs differ more in leaf ca:mg than in tolerance of low N, low P, or heavy metals. Plant Soil 280:49–64. https://doi.org/10.1007/s11104-005-3502-y
Oze C, Skinner C, Schroth AW, Coleman RG (2008) Growing up green on serpentine soils: biogeochemistry of serpentine vegetation in the central coast range of California. Appl Geochem 23:3391–3403. https://doi.org/10.1016/j.apgeochem.2008.07.014
Pandolfini T, Pancaro L (1992) Biogeochemical survey of some ophiolitic outcrops in Tuscany. Flora 187:341–351
Pędziwiatr A (2015) Serpentinite in the Śnieżnik massif: petrology and ecological impact. Geosci Rec 1:21–26. https://doi.org/10.1515/georec-2015-0003
Proctor J (1970) Magnesium as toxic element. Nature 227:742–743. https://doi.org/10.1038/227742a0
Proctor J, Nagy L (1991) Ultramafic rocks and their vegetation: an overview. In: Baker AJM, Proctor J, Reeves RD (eds) The vegetation of ultramafic (serpentine) soils. Intercept, Andover, pp 469–494
Quantin C, Ettler V, Garnier J, Šebek O (2008) Sources and extractibility of chromium and nickel in soil profiles developed on Czech serpentinites. Comptes Rendus Geosci 340:872–882. https://doi.org/10.1016/j.crte.2008.07.013
Ratié G, Jouvin D, Garnier J, Rouxel O, Miska S, Guimarães E, Cruz Vieira L, Sivry Y, Zelano I, Montarges-Pelletier E, Thil F, Quantin C (2015) Nickel isotope fractionation during tropical weathering of ultramafic rocks. Chem Geol 402:68–76. https://doi.org/10.1016/j.chemgeo.2015.02.039
Ratié G, Quantin C, Jouvin D, Calmels D, Ettler V, Sivry Y, Vieira LC, Ponzevera E, Garnier J (2016) Nickel isotope fractionation during laterite Ni ore smelting and refining: implications for tracing the sources of Ni in smelter-affected soils. Appl Geochem 64:136–145. https://doi.org/10.1016/j.apgeochem.2015.09.005
Reeves RD (1992) Hyperaccumulation of nickel by serpentine plants. In: Baker AJM, John P, Reeves RD (eds) The vegetation of ultramafic (serpentine) soils. Intercept, Andover, pp 253–277
Reeves RD, Baker AJM (2000) Metal-accumulating plants. In: Raskin I, Ensley, Burt D (eds) phytoremediation of toxic metals. Using plants to clean up the environment. Wiley, New York, pp 193–229
Reeves RD, Baker AJM, Becquer T, Echevarria G, Miranda ZJG (2007a) The flora and biogeochemistry of the ultramafic soils of Goiás state, Brazil. Plant Soil 293:107–119. https://doi.org/10.1007/s11104-007-9192-x
Reeves RD, Baker AJM, Romero R (2007b) The ultramafic flora of the Santa Elena peninsula, Costa Rica: a biogeochemical reconnaissance. J Geochemical Explor 93:153–159. https://doi.org/10.1016/j.gexplo.2007.04.002
Rencz AN, Shilts WW (1990) Nickel in soils and glaciated terrains. In: Nriagau JO (ed) Nickel in the environment. Wiley, New York, pp 151–188
Robinson BH, Brooks RR, Clothier BE (1999) Soil amendments affecting nickel and cobalt uptake by Berkheya coddii: potential use for Phytomining and phytoremediation. Ann Bot 84:689–694. https://doi.org/10.1006/anbo.1999.0970
Samecka-Cymerman A, Garbiec K, Kolon K, Kempers AJ (2008) Factor analysis of the elemental composition of Pteridium Aquilinum from serpentine and granite soils as a tool in the classification of relations between this composition and the type of parent rock in the Ślęża massif in lower Silesia, Poland. Environ Geol 58:509–514. https://doi.org/10.1007/s00254-008-1524-5
Seregin IV, Kozhevnikova AD (2006) Physiological role of nickel and its toxic effects on higher plants. Russ J Plant Physiol 53:257–277. https://doi.org/10.1134/S1021443706020178
Shewry PR, Peterson PJ (1971) Distribution of chromium and nickel in plants and soil from serpentine and other sites. J Ecol 64:195–212
Siebert C, Nagler TF, Kramers JD (2001) Determination of molybdenum isotope fractionation by double-spike multicollector inductively coupled plasma mass spectrometry. Geochem Geophys Geosyst 2:2000GC0000124
Šillerová H, Chrastný V, Vítková M, Francová A, Jehlička J, Gutsch MR, Kocourková J, Aspholm PE, Nilsson LO, Berglen TF, Jensen HKB, Komárek M (2017) Stable isotope tracing of Ni and cu pollution in north-East Norway: potentials and drawbacks. Environ Pollut. https://doi.org/10.1016/j.envpol.2017.05.030
StatSoft I (2011) Statistica (data analysis software system)
Szczęśniak E (2003) Rare and threatened species of thermophilous swards in Lower Silesia. In: Kącki Z (ed) Endangered vascular plants of Lower Silesia. Instytut Bioogii Roślin Uniwersytetu Wrocławskiego,Polskie Towarzystwo Przyjaciół Przyrody “Pro Natura,” Wrocław, pp 85–107. in Polish
Trepka S, Mierzejewski M (1957) The geological map of the Sudety Mountains - Jordanów Śląski. Geological Institute, UK
Tutin TG, Heywood VH, Burges NA, Moore DM, Valentine DH, Walters SM, Webb DA (1968) Flora Europaea. Volume 2: Rosaceae to Umbelliferae. Cambridge University press, Cambridge
Tutin TG, Heywood VH, Burges NA, Moore DM, Valentine DH, Walters SM, Webb DA (1972) Flora Europaea. Volume 3: Diapensiaceae to Myoporaceae. Cambridge University press, Cambridge
Tutin TG, Heywood VH, Burges NA, Valentine DH, Walters SM, Webb DA (1976) Flora Europaea. Volume 4: Plantaginaceae to Compositae (and Rubiaceae). Cambridge University press, Cambridge
Tutin TG, Heywood VH, Burges NA, Moore DM, Valentine DH, Walters SM, Webb DA (1980) Flora Europaea. Volume 5: Alismataceae to Orchidaceae (Monocotyledones). Cambridge University press, Cambridge
Tutin TG, Burges NA, Chater AO, Edmondson JR, Heywood VH, Moore DM, Valentine DH, Walters SM, Webb DA (1993) Flora Europaea. Volume 1:Psilotaceae to Platanaceae., 2nd edn. Cambridge University press, Cambridge
Uren NC (1992) Forms, reactions and availability of nickel in soils. Adv Agron 48:141–203
Van der Ent A, Baker AJM, Reeves RD, Pollard JA, Schat H (2013a) Hyperaccumulators of metal and metalloid trace elements: facts and fiction. Plant Soil 362:319–334. https://doi.org/10.1007/s11104-012-1287-3
Van der Ent A, Baker AJM, van Balgooy MMJ, Tjoa A (2013b) Ultramafic nickel laterites in Indonesia (Sulawesi, Halmahera): mining, nickel hyperaccumulators and opportunities for phytomining. J Geochemical Explor 128:72–79. https://doi.org/10.1016/j.gexplo.2013.01.009
Van der Ent A, Rajakaruna N, Boyd R, Echevarria G, Repin R, Williams D (2015) Global research on ultramafic (serpentine) ecosystems (8th international conference on serpentine ecology in Sabah, Malaysia). Aust J Bot 63:1–16. https://doi.org/10.1071/BTv63n4_IN
Van der Ent A, Callahan DL, Noller BN, Mesjasz-Przybylowicz J, Przybylowicz WJ, Barnabas A, Harris HH (2017) Nickel biopathways in tropical nickel hyperaccumulating trees from Sabah (Malaysia). Sci Rep 7:41861. https://doi.org/10.1038/srep41861
Van der Ent A, Cardace D, Tibbett M, Echevarria G (2018) Ecological implications of pedogenesis and geochemistry of ultramafic soils in Kinabalu Park ( Malaysia ). Catena 160:154–169. https://doi.org/10.1016/j.catena.2017.08.015
Wang P, Zhou D-M, Peijnenburg WJGM, Li LZ, Weng N (2010) Evaluating mechanisms for plant-ion (Ca2+, Cu2+, Cd2+ or Ni2+) interactions and their effectiveness on rhizotoxicity. Plant Soil 334:277–288. https://doi.org/10.1007/s11104-010-0381-7
Waroszewski J, Egli M, Kabala C, Kierczak J, Brandova D (2016) Mass fluxes and clay mineral formation in soils developed on slope deposits of the Kowarski Grzbiet (Karkonosze Mountains, Czech Republic/Poland). Geoderma 264:363–378. https://doi.org/10.1016/j.geoderma.2015.08.044
Weber J (1980) Genesis and properties of soils derived from serpentinites in Lower Silesia.Characteristics of parent material. Rocz Glebozn XXXI:143–161. in Polish
White PJ (2000) Calcium channels in higher plants. Biochim Biophys Acta Biomembr 1465:171–189. https://doi.org/10.1016/S0005-2736(00)00137-1
Whittaker RH (1954) The ecology of serpentine soils. Ecology 35:258–288
Wilson MJ (2004) Weathering of the primary rock-forming minerals : processes , products and rates. Clay Miner 39:233–266. https://doi.org/10.1180/0009855043930133
Yadav SK (2010) Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. South African J Bot 76:167–179. https://doi.org/10.1016/j.sajb.2009.10.007
Yusuf M, Fariduddin Q, Hayat S, Ahmad A (2011) Nickel: an overview of uptake, essentiality and toxicity in plants. Bull Environ Contam Toxicol 86:1–17. https://doi.org/10.1007/s00128-010-0171-1
Zelano IO, Cloquet C, Echevarria G, Van der Ent A, Fraysse F, Montarges-Pelletier E (2017) Nickel isotopic fractionation and the role of Hyperaccumulating plants. Goldschmidt Conference, Paris, 13–18 (August)
Żołnierz L (2007) Grasslands on serpentines in lower Silesia (SW Poland) - some aspects of their ecology. Wydawnictwo Uniwersytetu Przyrodniczego we Wrocławiu, Wrocław in Polish
Acknowledgements
This research was financed by Polish National Science Center (Jakub Kierczak, project No 2012/ 05/D/ST10/00529). Artur Pędziwiatr is grateful for Human Capital Operating Program of European Union (project No POKL.04.01.01-00-054/10-00). The authors would like to thank Jerzy Raczyk and Gaël Monvoisin for support during chemical analysis. We are grateful also to Olivier Rouxel for the Ni double spike preparation, as well as to Agnieszka Koczur and Anna Pietranik for valuable comments for the manuscript. The authors wish to thank the three anonymous Reviewers, which allowed us to significantly improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Antony Van der Ent.
Electronic supplementary material
Online Resource 1.
Nickel, Cr, and Co concentrations in leachates from the DTPA-CaCl2 and EDTA extractions. (DOCX 17 kb)
Online Resource 2.
Phytosociological affiliation of studied plants. (DOCX 18 kb)
Online Resource 3.
Results of the U-Mann Whitney test and the Cochran-Cox test (α = 0.05). (XLSX 11 kb)
Online Resource 4.
Spearman rank correlation or Pearson linear correlation (α = 0.05). (XLSX 14 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Pędziwiatr, A., Kierczak, J., Waroszewski, J. et al. Rock-type control of Ni, Cr, and Co phytoavailability in ultramafic soils. Plant Soil 423, 339–362 (2018). https://doi.org/10.1007/s11104-017-3523-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-017-3523-3