Abstract
Aims
The below-canopy soil moisture content and litter-layer arthropod abundance and diversity of Acacia karroo trees parasitized by each of three mistletoe species (Erianthemum ngamicum, Plicosepalus kalachariensis, and Viscum verrucosum) and uninfected A. karroo trees were investigated in semi-arid savanna, southwest Zimbabwe.
Results
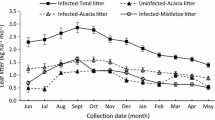
The soils below the canopies of mistletoe-infected trees were significantly low in moisture content compared to those beneath uninfected A. karroo trees. Nevertheless, arthropod species diversity was greater by up to 34 % below the canopies of mistletoe-infected trees than beneath uninfected A. karroo trees, with greater abundances beneath trees infected by E. ngamicum and P. kalachariensis. In addition, the majority of the arthropod species associated with mistletoe-infected trees had litter as their dominant foraging substrate.
Conclusions
Our findings show that mistletoes increase the abundance and diversity of litter-dwelling and –foraging arthropods due to increase in the quality and quantity of litterfall beneath mistletoe-infected trees. By altering the below-canopy arthropod communities and soil moisture content, mistletoes have potential to modify ecosystem processes such as decomposition, soil process rates, and nutrient cycling. Therefore, we suggest that the resulting increase in resource heterogeneity plays an important role in determining the structure and functioning of semi-arid savanna ecosystems.



Similar content being viewed by others
References
Anderson SJ, Braby M (2009) Invertebrate diversity associated with tropical mistletoe in a suburban landscape from northern Australia. North Territ Nat 21:2–23
Barberena-Arias MF, González G, Cuevas E (2012) Quantifying variation of soil arthropods using different sampling protocols: is diversity affected? In: Sudarshana P, Nageswara-Rao M, Soneji JR (eds) Tropical Forest. InTech Online Publisher, www.intechopen.com, pp. 51–70
Bardgett RD (2005) The Biology of Soil: A Community and Ecosystem. Oxford University Press, Oxford
Bultman TL, Uetz GW (1984) Effect of structure and nutritional quality of litter on abundances of litter-dwelling arthropods. Am Midl Nat 111:165–172
Burns AE, Cunningham SA, Watson DM (2011) Arthropod communities in tree canopies: an ordinal comparison between assemblages on mistletoes and their eucalypt hosts. Aust J Entomol 50:221–230
Canyon DV, Hill CJ (1997) Mistletoe host-resemblance: a study of herbivory, nitrogen and moisture in two Australian mistletoes and their host trees. Aust J Ecol 22:395–403
Cullings K, Hanely J (2010) Dwarf mistletoe effects on soil basidomycete community structure, soil fungal functional diversity, and soil enzyme function: implications for climate change. Soil Biol Biochem 42:1976–1981
Cullings K, Raleigh C, Vogler DR (2005) Effects of dwarf mistletoe infection on the ectomycorrhizal community of a Pinus contorta stand in Yellowstone Park. Can J Bot 83:1174–1180
Dye PJ (1983) Prediction of variation in grass growth in a semi-arid induced grassland. PhD thesis, University of the Witswatersrand, Johannesburg, South Africa
Dye PJ, Walker BH (1987) Patterns of shoot growth in a semi-arid grassland in Zimbabwe. J App Ecol 24:633–644
Ehleringer JR, Marshall JD (1995) Water relations. In: Press MC, Graves DJ (eds) Parasitic plants. Chapman and Hall, London, pp 125–140
Holt JA, Coventry RJ (1990) Nutrient cycling in Australian savannas. J Biogeogr 17:427–432
Irish J (1992) The Hetrodinae (Orthoptera: Enisfera: Bradyporidae) of Southern Africa: Systematics and Phylogeny. Navors Nas Mus Bloemfontein 8:394–434
Kareiva P (1987) Habitat fragmentation and the stability of predator–prey interactions. Nature 326:388–390
Krebs CJ (1999) Ecological methodology. Benjamin Cummings, Menlo Park
Kuijt J (1969) The biology of parasitic flowering plants. University of California Press, Berkeley
Malaka SLO (1996) Termites in West Africa. University of Lagos Press, Lagos
Mapaura A, Timberlake J (2004) A checklist of Zimbabwean vascular plants. Southern African Botanical Diversity Network Report No. 33. SABONET, Pretoria and Harare
March WA (2007) The impact of an Australian mistletoe, Amyema miquelii (Loranthaceae), on nutrient cycling in eucalypt forests and woodlands (PhD Thesis). Charles Sturt University, Thurgoona
March WA, Watson DM (2007) Parasites boost productivity: effects of mistletoe on litterfall dynamics in a temperate Australian forest. Oecologia 154:339–347
March WA, Watson DM (2010) The contribution of mistletoes to nutrient returns: evidence for a critical role in nutrient cycling. Aust Ecol 35:713–721
Mueller RC, Gehring CA (2006) Interactions between an above-ground plant parasite and below-ground ectomycorrhizal fungal communities on pinyon pine. J Ecol 94:276–284
Ndagurwa HGT, Dube JS, Mlambo D (2013) The influence of mistletoes on nitrogen cycling in a semi-arid savanna, southwest Zimbabwe. J Trop Ecol 29:147–159
Ndagurwa HGT, Dube JS, Mlambo D, Mlambo D (2014) The influence of mistletoes on nutrient cycling in a semi-arid savanna, southwest Zimbabwe. Plant Ecol 215:15–26
Ndagurwa HGT, Mundy PJ, Dube JS, Mlambo D (2012) Patterns of mistletoe infection in four Acacia species in a semi-arid southern African savanna. J Trop Ecol 28:523–526
Picker M, Griffiths C, Weaving A (2004) Field Guide to Insects of South Africa. Struik, South Africa
Press MC (1998) Dracula or Robin Hood? A functional role of root hemiparasites in nutrient poor ecosystems. Oikos 82:609–611
Press MC, Phoenix GK (2005) Impacts of parasitic plants on natural communities. New Phytol 166:737–751
Rattray JM (1957) The grasses and grass associations of southern Rhodesia. Rhod Agric J 54:197–234
Razeng E, Watson DM (2012) What do declining woodland birds eat? A synthesis of dietary records. Emu 112:149–159
Room PM (1972a) The constitution and natural history of the fauna of the mistletoe Tapinanthus bangwensis (Engl. & K. Krause) growing on cocoa in Ghana. J Anim Ecol 41:519–535
Room PM (1972b) The fauna of the mistletoe Tapinanthus bangwensis (Engl. & K. Krause) growing on cocoa in Ghana: relationships between fauna and mistletoe. J Anim Ecol 41:611–621
Sala A, Carrey EV, Callaway RM (2001) Dwarf mistletoe affects whole-tree water relations of Douglas fir and western larch primarily through changes in leaf to sapwood ratios. Oecologia 126:42–52
Scholtz CH, Holm E (1985) Insects of Southern Africa. Butterworths, Durban
Schuurman G (2005) Decomposition rates and termite assemblage composition in semiarid Africa. Ecology 86:1236–1249
Seastedt TR, Crossley DA (1981) Microarthropod response following cable logging and clear-cutting in the southern Appalachians. Ecology 62:126–135
Shaw DC, Watson DM, Mathiason RL (2004) Comparison of dwarf mistletoes (Arceuthobium spp., Viscaceae) in the western United States with mistletoes (Amyema spp., Loranthaceae) in Australia – ecological analogs and reciprocal models for ecosystem management. Aust J Bot 52:481–498
Skaife SH (1979) African insect life. Struik, Cape Town
Swift MJ, Heal OW, Anderson JM (1979) Decomposition in Terrestrial Ecosystems. University of California Press, Berkeley
Van der Putten WH, Van Dijk C, Peters BAM (1993) Plant-specific soil-borne diseases contribute to succession in foredune vegetation. Nature 362:53–56
Ward HK, Richardson FD, Denny RP, Dye PT (1979) Matopos research station: a perspective. Rhod Agric J 76:5–18
Wardle DA (2002) Communities and ecosystems. Linking the aboveground and belowground components. Princeton University Press, Princeton, p 57
Watson DM (2001) Mistletoe—a keystone resource in forests and woodlands worldwide. Ann Rev Ecol Syst 32:219–249
Watson DM (2002) Effects of mistletoe on diversity: a casestudy from southern New South Wales. Emu 102:275–281
Watson DM (2009) Parasitic plants as facilitators: more Dryad than Dracula? J Ecol 97:1151–1159
Watson DM (2011) A productivity-based explanation for woodland bird declines: poorer soils yield less food. Emu 111:10–18
Watson DM, Herring M (2012) Mistletoe as a keystone resource: an experimental test. Proc R Soc B 279:3853–3860
Watson DM, McGregor HW, Spooner PG (2009) Hemiparasitic shrubs increase resource availability and multi-trophic diversity of eucalypt forest birds. Funct Ecol 150:889–899
Whittake PL (1982) Community ecology of Phoradendron tomentosum in southern Texas. PhD thesis, University of Texas, Austin, Texas
Acknowledgments
This research was supported by a grant from the National University of Science and Technology Research Board (Grant RB/11/10). We are grateful to the people who assisted with fieldwork, particularly Alford Magwizi, Kudzanai Dhliwayo, and Obey Sonono. Laboratory work was carried out at the Natural History Museum of Zimbabwe, and were are grateful to the Department of Entomology specifically, Kudzai Mafuwe and staff. We also thank David M Watson and another anonymous reviewer for their time and effort that allowed us to increase the clarity and quality of our work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Duncan D. Cameron.
Rights and permissions
About this article
Cite this article
Ndagurwa, H.G.T., Dube, J.S., Mlambo, D. et al. The influence of mistletoes on the litter-layer arthropod abundance and diversity in a semi-arid savanna, Southwest Zimbabwe. Plant Soil 383, 291–299 (2014). https://doi.org/10.1007/s11104-014-2176-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-014-2176-8