Abstract
Purpose
Refractory prolactinomas resistant to dopamine agonists (DAs) pose a clinical challenge. Temozolomide (TMZ) is a recommended treatment option, but its effects are difficult to predict, and the alternatives are limited. Recent reports suggested that TMZ combined with capecitabine (CAPTEM) can be effective for the treatment of aggressive pituitary tumors. This study sought to evaluate the effect of TMZ in an ex vivo three-dimensional (3D) spheroid culture assay and determine if this assay could be used to predict the therapeutic effect of CAPTEM in actual refractory prolactinomas.
Methods
Surgically resected tumor tissues from two patients with refractory prolactinoma were cultured as 3D spheroids. The effects of TMZ were assessed based on its suppression of cell viability and reduction of prolactin (PRL) levels.
Results
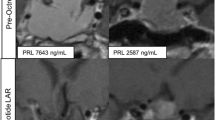
In Case 1, the 3D culture assay showed no effect of TMZ on cell viability or PRL suppression. Clinically, TMZ treatment did not reduce PRL levels (8870→8274 ng/mL) and the tumor progression. However, CAPTEM partially reduced PRL levels (9070→4046 ng/mL) and suppressed the tumor growth. In Case 2, TMZ in the 3D culture assay showed a 50% reduction of cell viability and 40% reduction of PRL levels. Clinically, CAPTEM was highly effective, with a considerable reduction in PRL level (17,500→210 ng/mL), and MRI showed almost no residual tumor.
Conclusions
This is the first report to describe the effects of CAPTEM treatment on refractory prolactinomas. The ex vivo 3D spheroid culture assay reliably predicted TMZ sensitivity and informed the selection between TMZ or CAPTEM treatment for refractory prolactinomas.


Similar content being viewed by others
References
Di Sarno A, Landi ML, Cappabianca P et al (2001) Resistance to cabergoline as compared with bromocriptine in hyperprolactinemia: prevalence, clinical definition, and therapeutic strategy. J Clin Endocrinol Metab 86(11):5256–5261
Delgrange E, Daems T, Verhelst J, Abs R, Maiter D (2009) Characterization of resistance to the prolactin-lowering effects of cabergoline in macroprolactinomas: A study in 122 patients. Eur J Endocrinol 160:747–752
Lasolle H, Ilie MD, Raverot G (2020) Aggressive prolactinomas: How to manage? Pituitary 23(1):70–77
Liu W, Zahr RS, McCartney S, Cetas JS, Dogan A, Fleseriu M (2018) Clinical outcomes in male patients with lactotroph adenomas who required pituitary surgery: A retrospective single center study. Pituitary 21(5):454–462
Tang H, Cheng Y, Huang J, Li J, Zhang B, Wu ZB (2021) Case Report: Temozolomide treatment of refractory prolactinoma resistant to dopamine agonists. Front Endocrinol (Lausanne) 12:616339
Almalki MH, Aljoaib NN, Alotaibi MJ et al (2017) Temozolomide therapy for resistant prolactin-secreting pituitary adenomas and carcinomas: A systematic review. Hormones (Athens) 16(2):139–149
Nakano-Tateno T, Lau KJ, Wang J et al (2021) Multimodal non-surgical treatments of aggressive pituitary tumors. Front Endocrinol (Lausanne) 12:624686
McCormack A, Dekkers OM, Petersenn S et al (2018) Treatment of aggressive pituitary tumours and carcinomas: Results of a European Society of Endocrinology (ESE) survey 2016. Eur JEndocrinol 178:265–276
Zhang D, Way JS, Zhang X et al (2019) Effect of everolimus in treatment of aggressive prolactin-secreting pituitary adenomas. J Clin Endocrinol Metab 104(6):1929–1936
Cooper O, Bonert VS, Rudnick J et al (2021) EGFR/ErbB2-targeting lapatinib therapy for aggressive prolactinomas. J Clin Endocrinol Metab 106(2):e917–e925
Duhamel C, Ilie MD, Salle H et al (2020) Immunotherapy in corticotroph and lactotroph aggressive tumors and carcinomas: Two case reports and a review of the literature. J Pers Med 10(3):88
Lu Y, Zhao Z, Wang J et al (2018) Safety and efficacy of combining capecitabine and temozolomide (CAPTEM) to treat advanced neuroendocrine neoplasms: A meta-analysis. Med (Baltim) 97(41):e12784
Nakano-Tateno T, Satou M, Inoshita N et al (2020) Effects of CAPTEM (capecitabine and temozolomide) on a corticotroph carcinoma and an aggressive corticotroph tumor. Endocr Pathol 32:418–426
Zacharia BE, Gulati AP, Bruce JN et al (2014) High response rates and prolonged survival in patients with corticotroph pituitary tumors and refractory Cushing disease from capecitabine and temozolomide (CAPTEM): A case series. Neurosurgery 74(4):E447–E455
Syro LV, Rotondo F, Camargo M, Ortiz LD, Serna CA, Kovacs K (2018) Temozolomide and pituitary tumors: Current understanding, unresolved issues, and future directions. Front Endocrinol (Lausanne) 9:318
Burman P, Lamb L, McCormack A (2020) Temozolomide therapy for aggressive pituitary tumours - current understanding and future perspectives. Rev Endocr Metab Disord 21(2):263–276
Vlachogiannis G, Hedayat S, Vatsiou A et al (2018) Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 359(6378):920–926
Tuveson D, Clevers H (2019) Cancer modeling meets human organoid technology. Science 364(6444):952–955
Lee SY (2016) Temozolomide resistance in glioblastoma multiforme. Genes Dis 3(3):198–210
Murakami M, Mizutani A, Asano S et al (2011) A mechanism of acquiring temozolomide resistance during transformation of atypical prolactinoma into prolactin-producing pituitary carcinoma: case report. Neurosurgery 68(6):E1761–E1767
Matsuno A, Murakami M, Hoya K et al (2014) Molecular status of pituitary carcinoma and atypical adenoma that contributes the effectiveness of temozolomide. Med Mol Morphol 47(1):1–7
Tsujimoto Y, Shichi H, Fukuoka H et al (2021) Tumor shrinkage by metyrapone in cushing disease exhibiting glucocorticoid-induced positive feedback. J Endocr Soc 5(6):143664
Janssen JM, Jacobs BAW, Roosendaal J et al (2021) Population pharmacokinetics of intracellular 5-Fluorouridine 5’-Triphosphate and its relationship with hand-and-foot syndrome in patients treated with capecitabine. AAPS J 23(1):23
Ishida A, Asakuno K, Kato M et al (2021) Treatment of an anterior cerebral artery pseudoaneurysm secondary to a transsphenoidal surgery using stent-assisted coiling. Surg Neurol Int 12:20
Bengtsson D, Schrøder HD, Andersen M et al (2015) Long-term outcome and MGMT as a predictive marker in 24 patients with atypical pituitary adenomas and pituitary carcinomas given treatment with temozolomide. J Clin Endocrinol Metab 100(4):1689–1698
Raverot G, Burman P, McCormack A et al (2018) European Society of Endocrinology. European Society of Endocrinology Clinical Practice Guidelines for the management of aggressive pituitary tumours and carcinomas. Eur J Endocrinol 178(1):G1–G24
Lasolle H, Cortet C, Castinetti F et al (2017) Temozolomide treatment can improve overall survival in aggressive pituitary tumors and pituitary carcinomas. Eur J Endocrinol 176(6):769–777
Hirohata T, Asano K, Ogawa Y et al (2013) DNA mismatch repair protein (MSH6) correlated with the responses of atypical pituitary adenomas and pituitary carcinomas to temozolomide: the national cooperative study by the Japan Society for Hypothalamic and Pituitary Tumors. J Clin Endocrinol Metab 98(3):1130–1136
Losa M, Bogazzi F, Cannavo S et al (2016) Temozolomide therapy in patients with aggressive pituitary adenomas or carcinomas. J Neurooncol 126(3):519–525
Bush ZM, Longtine JA, Cunningham T et al (2010) Temozolomide treatment for aggressive pituitary tumors: correlation of clinical outcome with O(6)-methylguanine methyltransferase (MGMT) promoter methylation and expression. J Clin Endocrinol Metab 95(11):E280–E290
Yip S, Miao J, Cahill DP et al (2009) MSH6 mutations arise in glioblastomas during temozolomide therapy and mediate temozolomide resistance. Clin Cancer Res 15(14):4622–4629
Micko ASG, Wöhrer A, Höftberger R et al (2017) MGMT and MSH6 immunoexpression for functioning pituitary macroadenomas. Pituitary 20(6):643–653
Breslin S, O’Driscoll L (2013) Three-dimensional cell culture: the missing link in drug discovery. Drug Discov Today 18(5–6):240–249
Campderá M, Palacios N, Aller J et al (2016) Temozolomide for aggressive ACTH pituitary tumors: failure of a second course of treatment. Pituitary 19(2):158–166
Lin AL, Jonsson P, Tabar V et al (2018) Marked Response of a Hypermutated ACTH-Secreting Pituitary Carcinoma to Ipilimumab and Nivolumab. J Clin Endocrinol Metab 103(10):3925–3930
Kulke MH, Hornick JL, Frauenhoffer C et al (2009) O6-methylguanine DNA methyltransferase deficiency and response to temozolomide-based therapy in patients with neuroendocrine tumors. Clin Cancer Res 15(1):338–345
Murakami J, Lee YJ, Kokeguchi S et al (2007) Depletion of O6-methylguanine-DNA methyltransferase by O6-benzylguanine enhances 5-FU cytotoxicity in colon and oral cancer cell lines. Oncol Rep 17(6):1461–1467
Lasolle H, Vasiljevic A, Borson-Chazot F, Raverot G (2019) Pasireotide: a potential therapeutic alternative for resistant prolactinoma. Ann Endocrinol 80:84–88
Acknowledgements
We would like to thank Drs. Hideki Shiramizu, Haruko Yoshimoto, Masataka Kato, Nobuhiro Miki, Masami Ono for discussion.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no multiplicity of interests to disclose.
Ethical approval
Study protocols were approved by the Moriyama Memorial Hospital Institutional Review Board. Informed consent was obtained from the patients. 3D spheroid culture experiments were conducted in compliance with the protocol that was reviewed and approved by the Research Ethics Committee of Kobe University Hospital (IRB#1363).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ishida, A., Shichi, H., Fukuoka, H. et al. Efficacy of temozolomide combined with capecitabine (CAPTEM) on refractory prolactinomas as assessed using an ex vivo 3D spheroid assay. Pituitary 25, 238–245 (2022). https://doi.org/10.1007/s11102-021-01192-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-021-01192-x