Abstract
Purpose
Surgical workflow analysis seeks to systematically break down operations into hierarchal components. It facilitates education, training, and understanding of surgical variations. There are known educational demands and variations in surgical practice in endoscopic transsphenoidal approaches to pituitary adenomas. Through an iterative consensus process, we generated a surgical workflow reflective of contemporary surgical practice.
Methods
A mixed-methods consensus process composed of a literature review and iterative Delphi surveys was carried out within the Pituitary Society. Each round of the survey was repeated until data saturation and > 90% consensus was reached.
Results
There was a 100% response rate and no attrition across both Delphi rounds. Eighteen international expert panel members participated. An extensive workflow of 4 phases (nasal, sphenoid, sellar and closure) and 40 steps, with associated technical errors and adverse events, were agreed upon by 100% of panel members across rounds. Both core and case-specific or surgeon-specific variations in operative steps were captured.
Conclusions
Through an international expert panel consensus, a workflow for the performance of endoscopic transsphenoidal pituitary adenoma resection has been generated. This workflow captures a wide range of contemporary operative practice. The agreed “core” steps will serve as a foundation for education, training, assessment and technological development (e.g. models and simulators). The “optional” steps highlight areas of heterogeneity of practice that will benefit from further research (e.g. methods of skull base repair). Further adjustments could be made to increase applicability around the world.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Endonasal transsphenoidal approaches to the skull base are emerging as the first-line approach for resecting the majority of pituitary adenomas which require surgical intervention [1,2,3]. However, there is variation in the ways in which these operations are performed, largely based on surgeon preference and training, which may result in differing surgical outcomes [4,5,6,7]. These operations are technically demanding, relatively low volume, with steep learning curves—culminating in the frequent requirement for dedicated fellowships to achieve procedure-specific competency [8,9,10,11].
Surgical workflow analysis seeks to systematically break down surgical procedures into defined tasks and errors [12, 13]. In this hierarchical process, procedures are broken down into phases which contain a series of steps, generating a dedicated workflow [13]. During each step (e.g. suturing), surgical instruments (e.g. forceps) are used to perform manoeuvres (e.g. knot tying) via a series of gestures (e.g. grasping and pulling suture) [14]. Similarly, at each step, there is the potential for technical errors (lapses in surgical technique) and adverse events (an event that may lead to adverse outcomes or postoperative complications) [12].
These workflows may be used for the training (for example, creation of simulations), objective assessment of procedure-specific surgical skills and evaluation of novel surgical technologies or techniques [12, 15,16,17]. By creating a complimentary nurse and anaesthetic workflow analysis, operating room efficiency may be improved by orchestrating the surgical team [15]. The principal limitation to workflow analysis is the labelling and segmentation of operations into constituent phases, steps and errors, however this process can be automated (or semi-automated) using machine learning techniques [18,19,20]. The effectiveness of such automation is dependent on the generation of a comprehensive and exhaustive workflow to train deep neural networks to recognise the phases, steps, instruments and errors of an operation.
Consensus processes involving subject experts have been used in order to generate a comprehensive and standardised workflow for named operations [15, 21, 22]. The Delphi technique allows for the generation of group consensus through iterative surveys, interspersed with feedback [23]. Questions nested within surveys can be qualitative or quantitative (often using ordinal scales). If quantitative metrics are used, simplified scales (e.g. 3-point) may translate more clearly into clinical practice with greater test–retest reliability [24]. With an engaged group of experts and the use of digital technologies, the process can be achieved in an accelerated fashion (a matter of weeks) [25]. The management of pituitary adenomas has benefitted from consensus statements, with groups such as the Pituitary Society producing a number of guidelines through its multidisciplinary specialist network [26,27,28,29,30,31,32]. However, there is no consensus on the operative workflow for endonasal transsphenoidal approaches (TSA) to pituitary adenomas.
We, therefore, sought to generate a surgical workflow for endoscopic TSA resection of pituitary adenomas, via an expert consensus process nested within the Pituitary Society.
Methods
Overview
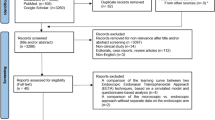
This process aimed to generate a surgical workflow that captured the range of ways the operation is performed in contemporary practice. The aim of the process was not to decide on the optimal set of surgical phases, steps or instruments—this will be explored in subsequent studies. In order to create this exhaustive workflow, expert input was derived through an iterative, mixed-methods consensus process (Fig. 1). The components of the workflow analysis and associated definitions are listed in Table 1 [13, 33]. The beginning of the operation was taken at entry of the endoscope endonasally with the use of surgical instruments, reflecting the American College of Surgeons definition of surgery—“structurally altering the human body by the incision or destruction of tissues” [34].
Modified Delphi process & sampling
Literature review
The process (Fig. 1) began with a brief literature review of neurosurgical textbooks and articles (PubMed or EMBASE). Keywords “endoscopic transsphenoidal”, “pituitary adenoma” and “operative technique” were used. From the relevant resources found, an initial operative workflow was generated [5,6,7, 35, 36].
Consensus round 1
The initial, literature-based workflow was discussed with a small group (n = 7) of experts—UK and Ireland based consultant neurosurgeon members of the Pituitary Society. Each expert reviewed the workflow individually—via computerised document (Microsoft Word, Version 16.4, Microsoft, Washington, USA)—with the definitions of phases, steps, instruments, technical errors and adverse events as above. Each expert was asked a series of questions (via e-mail), seeking to assess the completeness and accuracy of the workflow (“Appendix A” section). Any additional suggestions were reviewed and added to the workflow matrix if (i) in-scope, (ii) not duplicate. According to the Delphi technique, circulation and iterative revision of the workflow was repeated until data saturation was achieved, that is, all experts were satisfied that the workflow was complete and accurate. Resultantly, round 1 was repeated three times, occurring over 12 weeks (October 2020–Jan 2021).
Consensus round 2
The refined workflow was then sent to a larger group (n = 11)—international members of the Pituitary Society that are recognised experts in the field and nominated by the Physician Education Committee. Again, individuals were asked to assess the workflow (“Appendix A” section), and expand the defined domains (steps, instruments, technical errors and adverse events) to cover possible global variations in practice. As in Round 1, any additional suggestions were reviewed and added to the workflow matrix if (i) in-scope, (ii) not duplicate. This round was completed until (i) all experts agreed that the workflow captures the operative practice they have observed and (ii) there were no additional suggestions for the workflow from the participant group. Round 2 was repeated twice, occurring over 8 weeks (January 2021–March 2021).
Administration
Invitations to participate in the Delphi process were via direct email only. Workflow documents were presented using Microsoft Word (Version 16.4, Microsoft, Washington, USA) in both rounds and supported by Google Forms (Google LLC, California, USA) in Round 2.
Data collection and analysis
Participant demographics collected included training grade and country of practice. The collected data regarding the surgical workflow were quantitative (whether participants agree it is complete and accurate) and qualitative (additional suggestions or comments). Summary statistics (e.g. frequencies) were generated for participants demographics. Content analysis was used to analyse free-text responses—to remove out-of-scope suggestions, group similar suggestions together and compare them to existing data points in the workflow. Data analysis and workflow updates were performed in duplicate by two independent analysers (HJM, DZK).
Ethics
No identifiable data were collected about participants in the Delphi process. This study was independent of national health services and did not require ethical approval (interrogated via online Health Research Authority decision tool—“Appendix B” section) [37].
Results
General
There was a 100% response rate and no attrition across both Delphi rounds. Across both rounds, 18 panel members participated, representing seven countries: United Kingdom (n = 6), United States of America (n = 7), Australia (n = 1), Colombia (n = 1), Germany (n = 1), Italy (n = 1) and Republic of Ireland (n = 1).
Final surgical workflow
Four distinct operative phases were delineated on discussion—nasal, sphenoid, sellar and closure. The component steps within each phase were defined as core (necessary) or optional (case and/or surgeon dependent) and were agreed upon by 100% of panel members across rounds. Pre-operative set-up and post-operative protocols were judged as important but not included as per the defined study scope.
Nasal phase
This phase was composed of 10 steps (4 core, 6 optional), from the identification of pertinent nasal anatomy until entry into the sphenoid sinus (Table 2). Amongst our panel, this phase was performed both with otorhinolaryngologists or by neurosurgeons alone.
Sphenoid phase
This phase was the shortest in terms of the number of steps, composed of 4 steps (3 core, 1 optional) as detailed in Table 3.
Sellar phase
The sellar phase was composed of 12 steps (7 core, 5 optional) representing entry into the intracranial space and tumour (macroadenoma or microadenoma) resection (Table 4).
Closure phase
The closure phase was composed of 14 steps (3 core, 11 optional), consisting of haemostasis and repair of the skull base (when appropriate) (Table 5). This phase had the largest number of optional steps, reflecting the acknowledged heterogeneity in the various methods of skull base repair that may be used.
Discussion
Principal findings
Firstly, a workflow for the performance of endoscopic transsphenoidal pituitary adenoma resection has been generated, using Delphi methodology based on an international expert consensus agreement. The agreed “core” steps can be used for education (e.g. operative video annotation), surgical skills assessment, and the development of models and simulators [13, 19, 22, 38]. Similarly, the agreed “optional” steps highlight areas of heterogeneity of practice that will benefit from further research—most notably in skull base reconstruction (closure phase) and surgical exposure (nasal, sphenoid, sellar phases) [2, 3, 5, 7, 39]. This workflow also captures the instruments, errors and adverse events for each step and is the first of its kind in neurosurgery.
Furthermore, ensuring that the workflow captured a breadth of operative practice, in a structured fashion with consistent terminology, was a challenge and required multiple iterations across multiple rounds. For example, the presence of “optional” steps reflects differences between the practice of individual surgeons (e.g. choice of reparative material) and adaptation to case-specific factors (e.g. tumour extension) [5, 7, 40]. Resultantly, delineation of whether these steps were core or optional and the content of these steps (particularly instrument use) was an area of the workflow which required significant revisions. Similarly, another area that required significant iterative changes was distinguishing errors from adverse events and complications. Definitions of each of these components were therefore presented repeatedly, throughout each round. Adverse events were linked in line to particular technical errors and were limited to intra-operative consequences (as opposed to post-operative complications which occur later and more likely to be multifactorial) [33]. Many adverse events linked to particular technical errors were related to the damage of distinct anatomical structures (e.g. carotid artery) which often overlapped across adverse events with a step. Driven by consensus, the terminology was often broadened (e.g. “neurovascular injury, e.g. carotid artery injury”) to capture a breadth of events whilst decreasing repetition within steps and improving the readability of the workflow.
Findings in the context of existing literature
This Delphi consensus methodology has been used in various surgical specialities to generate similar surgical workflows, with demonstrated utility as a method to consolidate complex opinions into practical workflows [15, 17, 21, 22]. For example, a workflow for steps and errors in laparoscopic surgery by Bonrath et al. focussed on the need for standardised steps and errors for education and structured assessment of trainees [33]. Kaijser et al. explored the steps of laparoscopic bypass and sleeve gastrectomy in detail, deconstructing them further into constituent tasks in order to develop advanced simulators and training curricula [21]. Previous studies have tailored the workflow analysis to different levels of learners, for example, Dharamsi et al. highlighted the need and utility of a consensus-driven workflow for bougie-assisted cricothyroidotomy aimed specifically at novices [22]. A more in-depth analysis is occasionally performed to task or gesture level (which together make up a surgical step), and this level of granularity has been achieved through similar Delphi consensus techniques [41]. Notably, the terminology for the operative workflow hierarchy (e.g. phases, steps, tasks, gestures, motions) is not used in a standardised fashion (e.g. often task and step are used interchangeably) and alignment of future studies to a common language will be important as this field expands [13].
There are many applications of surgical workflows—including education and training; surgical assessment; research; and technology development. In relation to education, highlighting the core components of operations is a useful learning resource for training surgeons and has been used to develop educational curricula, courses and simulators [13, 38]. Similarly, these workflows can be used to inform objective assessment instruments specific to particular operations, for example, Knight et al. combined a consensus-driven surgical steps workflow for laparoscopic hysterectomy with an established skills assessment form (Objective structured assessment of technical skill or OSATS) to generate a reliable and specific measure of procedural proficiency [42]. Augmented assessment and training is particularly pertinent in low-volume surgeries, with steep learning curves and a unique set of surgical skills—such as pituitary surgery [8,9,10]. Resultantly, proficiency in such procedures requires dedicated fellowships and competency-based assessments, with services providing these operations becoming increasingly consolidated into centres of excellence [10, 26]. Operative workflows may facilitate this through standardisation of terminology, providing a platform to build education materials and specific skills assessments, and highlighting acceptable variations in contemporary practice [13].
A complimentary and related process to surgical workflow analysis is the segmentation of operative videos [13]. For example, focussing on laparoscopic colorectal surgery, Dijkstra et al. distilled the key operative steps—intending to use this information to segment operative videos into component steps [15]. These segmented videos are integrated into the intra-operative environment, to guide and assess trainee surgeons in a uniform fashion [15]. Indeed, such segmentation and procedure-specific analysis has been presented in live operations in animals, displaying an ability to improve the efficiency of tasks and reduce operative times [17]. A disadvantage of operative video segmentation is its labour-intensive nature, however, this process can be automated (or semi-automated) using machine learning techniques [18,19,20]. Indeed, in the context of the COVID-19 pandemic, where operative caseload is reduced (therefore maximising learning from each case is important) and waiting list backlog is at its highest (therefore more efficient surgery is important), these technologies may be particularly useful [43,44,45].
Strengths and limitations
There are several limitations to this study that are important to highlight. Whilst the Delphi method is useful for capturing and refining the opinions of various stakeholders, attention to expert panel selection will naturally influence process output [46]. In our study, our expert panel was international and multicentre. As expected, multicentre consensus processes are capable of identifying a broader and more granular workflow than single centre analyses [21, 47]. However, only one (of 18) expert panel members represented a low or middle-income country and thus our results may not reflect a global operative workflow for this procedure. Moreover, rating regarding the utility or rationale for operative steps (particularly optional steps) was not characterised in this study and this is certainly a point for further study. Finally, pre-operative set-up (e.g. nasal preparation and patient positioning) and post-operative strategies (e.g. placement of a nasogastric tube) were excluded for practical and scope purposes, and this again is an area that requires further study to characterise heterogeneity and explore comparative effectiveness.
Conclusions
Through an international expert panel consensus, a workflow for the performance of endoscopic transsphenoidal pituitary adenoma resection has been generated. This workflow captures a wide range of contemporary operative practice. The agreed “core” steps will serve as a foundation for education, training, assessment and technological development (e.g. models and simulators). The “optional” steps highlight areas of heterogeneity of practice that will benefit from further research (e.g. methods of skull base repair). Further adjustments could be made to increase applicability around the world.
Data availability
Available upon reasonable request.
Abbreviations
- eTSA:
-
Endoscopic Transsphenoidal Approach
- UK:
-
United Kingdom
- USA:
-
United States of America
- COVID-19:
-
Coronavirus Disease 2019
References
Cappabianca P, Cavallo LM, de Divitiis E (2004) Endoscopic endonasal transsphenoidal surgery. Neurosurgery 55(4):933–941
Liu JK, Das K, Weiss MH, Laws ER Jr., Couldwell WT (2001) The history and evolution of transsphenoidal surgery. J Neurosurg 95(6):1083–1096. https://doi.org/10.3171/jns.2001.95.6.1083
Couldwell WT, Weiss MH, Rabb C, Liu JK, Apfelbaum RI, Fukushima T (2004) Variations on the standard transsphenoidal approach to the sellar region, with emphasis on the extended approaches and parasellar approaches: surgical experience in 105 cases. Neurosurgery 55(3):539–550
Buchfelder M, Schlaffer S (2010) Pituitary surgery for Cushing’s disease. Neuroendocrinology 92(Suppl. 1):102–106
Lucas JW, Zada G (2012) Endoscopic surgery for pituitary tumors. Neurosurg Clin 23(4):555–569
Shah NJ, Navnit M, Deopujari CE, Mukerji SS (2004) Endoscopic pituitary surgery-a. beginner’s guide. Indian J Otolaryngol Head Neck Surg 56(1):71–78
Cappabianca P, Cavallo LM, de Divitiis O, Solari D, Esposito F, Colao A (2008) Endoscopic pituitary surgery. Pituitary 11(4):385–390
Leach P, Abou-Zeid AH, Kearney T, Davis J, Trainer PJ, Gnanalingham KK (2010) Endoscopic transsphenoidal pituitary surgery: evidence of an operative learning curve. Neurosurgery 67(5):1205–1212. https://doi.org/10.1227/NEU.0b013e3181ef25c5
Snyderman C, Kassam A, Carrau R, Mintz A, Gardner P, Prevedello DM (2007) Acquisition of surgical skills for endonasal skull base surgery: a training program. Laryngoscope 117(4):699–705
McLaughlin N, Laws ER, Oyesiku NM, Katznelson L, Kelly DF (2012) Pituitary centers of excellence. Neurosurgery 71(5):916–926
Jane JA, Sulton LD, Laws ER (2005) Surgery for primary brain tumors at United States academic training centers: results from the Residency Review Committee for neurological surgery. J Neurosurg 103(5):789–793
Sarker SK, Chang A, Albrani T, Vincent C (2008) Constructing hierarchical task analysis in surgery. Surg Endosc 22(1):107–111
Lalys F, Jannin P (2014) Surgical process modelling: a review. Int J Comput Assist Radiol Surg 9(3):495–511
Vedula SS, Malpani AO, Tao L, Chen G, Gao Y, Poddar P et al (2016) Analysis of the structure of surgical activity for a suturing and knot-tying task. PLoS ONE 11(3):e0149174
Dijkstra FA, Bosker RJI, Veeger NJGM, van Det MJ, Pierie JPEN (2015) Procedural key steps in laparoscopic colorectal surgery, consensus through Delphi methodology. Surg Endosc 29(9):2620–2627
Strauss G, Fischer M, Meixensberger J, Falk V, Trantakis C, Winkler D et al (2006) Workflow analysis to assess the efficiency of intraoperative technology using the example of functional endoscopic sinus surgery. HNO 54(7):528–535
Krauss A, Muensterer OJ, Neumuth T, Wachowiak R, Donaubauer B, Korb W et al (2009) Workflow analysis of laparoscopic Nissen fundoplication in infant pigs—a model for surgical feedback and training. J Laparoendosc Adv Surg Tech 19(S1):s117–s122
Twinanda AP, Shehata S, Mutter D, Marescaux J, De Mathelin M, Padoy N (2016) Endonet: a deep architecture for recognition tasks on laparoscopic videos. IEEE Trans Med Imaging 36(1):86–97
Lecuyer G, Ragot M, Martin N, Launay L, Jannin P (2020) Assisted phase and step annotation for surgical videos. Int J Comput Assist Radiol Surg 15:673–680
Zisimopoulos O, Flouty E, Luengo I, Giataganas P, Nehme J, Chow A et al (2018) Deepphase: surgical phase recognition in cataracts videos. Springer, Cham, pp 265–272
Kaijser MA, van Ramshorst GH, Emous M, Veeger N, van Wagensveld BA, Pierie JEN (2018) A Delphi consensus of the crucial steps in gastric bypass and sleeve gastrectomy procedures in the Netherlands. Obes Surg 28(9):2634–2643. https://doi.org/10.1007/s11695-018-3219-7
Dharamsi A, Gray S, Hicks C, Sherbino J, McGowan M, Petrosoniak A (2019) Bougie-assisted cricothyroidotomy: Delphi-derived essential steps for the novice learner. CJEM 21(2):283–290. https://doi.org/10.1017/cem.2018.386
Okoli C, Pawlowski SD (2004) The Delphi method as a research tool: an example, design considerations and applications. Inform Manag 42(1):15–29
Lange T, Kopkow C, Lützner J, Günther K-P, Gravius S, Scharf H-P et al (2020) Comparison of different rating scales for the use in Delphi studies: different scales lead to different consensus and show different test-retest reliability. BMC Med Res Methodol 20(1):28
Randic L, Carley S, Mackway-Jones K, Dunn K (2002) Planning for major burns incidents in the UK using an accelerated Delphi technique. Burns 28(5):405–412
Casanueva FF, Barkan AL, Buchfelder M, Klibanski A, Laws ER, Loeffler JS et al (2017) Criteria for the definition of Pituitary Tumor Centers of Excellence (PTCOE): a Pituitary Society Statement. Pituitary 20(5):489–498. https://doi.org/10.1007/s11102-017-0838-2
Lim EM, Pullan P (2005) Growth Hormone Research S, Pituitary S. Biochemical assessment and long-term monitoring in patients with acromegaly: statement from a joint consensus conference of the Growth Hormone Research Society and the Pituitary Society. Clin Biochem Rev 26(2):41–43
Arnaldi G, Angeli A, Atkinson AB, Bertagna X, Cavagnini F, Chrousos GP et al (2003) Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab 88(12):5593–5602
Ho K, Fleseriu M, Kaiser U, Salvatori R, Brue T, Lopes MB et al (2021) Pituitary neoplasm nomenclature workshop: does adenoma stand the test of time? J Endocr Soc 5(3):bvaa205
Fleseriu M, Buchfelder M, Cetas JS, Fazeli PK, Mallea-Gil SM, Gurnell M et al (2020) Pituitary society guidance: pituitary disease management and patient care recommendations during the COVID-19 pandemic—an international perspective. Pituitary 23(4):327–337
Giustina A, Barkhoudarian G, Beckers A, Ben-Shlomo A, Biermasz N, Biller B et al (2020) Multidisciplinary management of acromegaly: a consensus. Rev Endocr Metab Disord 21(4):667–678
Casanueva FF, Molitch ME, Schlechte JA, Abs R, Bonert V, Bronstein MD et al (2006) Guidelines of the Pituitary Society for the diagnosis and management of prolactinomas. Clin Endocrinol (Oxf) 65(2):265–273
Bonrath EM, Dedy NJ, Zevin B, Grantcharov TP (2014) International consensus on safe techniques and error definitions in laparoscopic surgery. Surg Endosc 28(5):1535–1544
Surgeons ACo: efinition of Surgery Legislative Toolkit (2007). Available at https://www.facs.org/-/media/files/advocacy/state/definition-of-surgery-legislative-toolkit.ashx#:~:text=Definition%20of%20Surgery%20Background&text=Surgery%20is%20performed%20for%20the,of%20the%20practice%20of%20medicine.. Accessed on 01 Mar 2021
Greenberg MS (2019) Handbook of neurosurgery, 9th edn. Georg Thieme Verlag, New York
Winn HR (2016) Youmans and Winn neurological surgery. Elsevier, Amsterdam
Health-Research-Authority: “Do I need NHS REC review?”. Available at http://www.hra-decisiontools.org.uk/research/ (2020). Accessed on 08 Sept 2020
Collins JW, Levy J, Stefanidis D, Gallagher A, Coleman M, Cecil T et al (2019) Utilising the Delphi process to develop a proficiency-based progression train-the-trainer course for robotic surgery training. Eur Urol 75(5):775–785. https://doi.org/10.1016/j.eururo.2018.12.044
Khan DZ, Bandyopadhyay S, Patel V, Schroeder BE, Cabrilo I, Choi D et al (2020) CSF rhinorrhoea after endonasal intervention to the anterior skull base (CRANIAL): proposal for a prospective multicentre observational cohort study. Br J Neurosurg. https://doi.org/10.1080/02688697.2020.1795622
Khan DZ, Marcus HJ, Horsfall HL, Bandyopadhyay S, Schroeder BE, Patel V et al (2021) CSF rhinorrhoea after endonasal intervention to the skull base (CRANIAL)-part 1: multicenter pilot study. World Neurosurg. https://doi.org/10.1093/bjs/znab135.034
Chen J, Oh PJ, Cheng N, Shah A, Montez J, Jarc A et al (2018) Use of automated performance metrics to measure surgeon performance during robotic vesicourethral anastomosis and methodical development of a training tutorial. J Urol 200(4):895–902
Knight S, Aggarwal R, Agostini A, Loundou A, Berdah S, Crochet P (2018) Development of an objective assessment tool for total laparoscopic hysterectomy: A Delphi method among experts and evaluation on a virtual reality simulator. PLoS ONE 13(1):e0190580
Carr A, Smith JA, Camaradou J, Prieto-Alhambra D (2021) Growing backlog of planned surgery due to covid-19. Br Med J Publ Group. https://doi.org/10.1136/bmj.n339
Davis CE, Hayes L, Dent N, Jennings I, Arumugasamy M, Walsh TN (2021) Impact of COVID-19 on surgical training. Br J Surg. https://doi.org/10.1093/bjs/znab057
Negopdiev D, Collaborative C, Hoste E (2020) Elective surgery cancellations due to the COVID-19 pandemic: global predictive modelling to inform surgical recovery plans. Br J Surg 107(11):1440–1449
Trevelyan EG, Robinson N (2015) Delphi methodology in health research: how to do it? Eur J Integr Med 7(4):423–428
van Rutte P, Nienhuijs SW, Jakimowicz JJ, van Montfort G (2017) Identification of technical errors and hazard zones in sleeve gastrectomy using OCHRA: “OCHRA for sleeve gastrectomy.” Surg Endosc 31(2):561–566. https://doi.org/10.1007/s00464-016-4997-4
Acknowledgements
This manuscript on behalf of the Professional Education Committee of the Pituitary Society, with special thanks to Pouneh K. Fazeli (Neuroendocrinology Unit, Division of Endocrinology and Metabolism, University of Pittsburgh, Pittsburgh, PA USA), Susana M. Mallea-Gil (Division de Endocrinología, Hospital Militar Central, Buenos Aires, Argentina), Ann McCormack (Department of Endocrinology, St Vincent’s Hospital, and Garvan Institute of Medical Research, Sydney, Australia), Maria M. Pineyro (Facultad de Medicina, Hospital de Clínicas, Clínica de Endocrinología Y Metabolismo, Universidad de La República, Montevideo, Uruguay), Nicholas A. Tritos (Neuroendocrine Unit and Neuroendocrine and Pituitary Tumor Clinical Center, Massachusetts General Hospital and Harvard Medical School, Boston, MA USA). HJM, DZK, CHK, HLH, WM are supported by the Wellcome (203145Z/16/Z) EPSRC (NS/A000050/1) Centre for Interventional and Surgical Sciences, University College London. HJM is also funded by the NIHR Biomedical Research Centre at University College London. MG is supported by the NIHR Cambridge Biomedical Research Centre. JWC receives research grants and consultancy fees from Medtronic (Dublin, Ireland). JWC is the associate medical director of CMR surgical (Cambridge, UK). DS is a shareholder in Odin Vision Ltd (London, UK) and is an employee of Digital Surgery (London, UK). This research was funded in whole, or in part, by the Wellcome Trust [203145Z/16/Z]. For the purpose of Open Access, the authors have applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
Funding
No specific funding was received for this piece of work.
Author information
Authors and Affiliations
Contributions
Study conception and methodology was led by HJM, JWC, MF, MG, DZK, CHK, HLH, WM. Investigation and data curation and resources were performed by AB, MB, JSC, NLD, MJ, PJ, ERL, AM, PM, NMO, THS, SS, GT, LVS, AW, MJW, GZ. Material preparation, data collection and analysis were performed by DZK and HJM. The first draft of the manuscript was written by DZK and HJM. All authors reviewed and edited subsequent versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
Ethical approval and informed consent were unnecessary due to the nature of the study (consensus process amongst health care professionals).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendices
Appendix A: guidance questions to experts during each consensus round
Round 1:
Q1. Do you think the presented workflow framework encapsulates your own operative practice and practice that you have observed?
If answered “No” to Q1:
Q2. Are there any additional operative steps which you feel should be added?
Q3. Are there any instruments used which are not represented in this framework? If so, at which step(s) would they be most appropriately place?
Q4. Are there any technical errors not listed in the framework? If so, at which step(s) would they be most appropriately place?
Q5. Are there any adverse events not listed in the framework? If so, at which step(s) would they be most appropriately place?
Round 2
A. Nasal Phase.
A1. Are there any additional operative steps which you feel should be added OR would you change any of the step contents?
A2. If yes, what would you change?
B. Sphenoid Phase.
B1. Are there any additional operative steps which you feel should be added OR would you change any of the step contents?
B2. If yes, what would you change?
C. Sellar Phase.
C1. Are there any additional operative steps which you feel should be added OR would you change any of the step contents?
C2. If yes, what would you change?
D. Closure Phase.
D1. Are there any additional operative steps which you feel should be added OR would you change any of the step contents?
D2. If yes, what would you change?
Appendix B: health research authority UK—Ethics requirement decision tool

Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marcus, H.J., Khan, D.Z., Borg, A. et al. Pituitary society expert Delphi consensus: operative workflow in endoscopic transsphenoidal pituitary adenoma resection. Pituitary 24, 839–853 (2021). https://doi.org/10.1007/s11102-021-01162-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-021-01162-3