Abstract
Purpose
Cushing’s disease (CD) is a severe illness generally caused by microcorticotropinomas (MICs) and in approximately 7–20% of patients by macrocorticotropinomas (MACs). USP8-mutations have been identified as a major genetic cause of CD (~ 50%). Few studies have reported the distribution between MICs–MACs related to USP8-mutations and their genotype–phenotype correlations. Therefore, we aimed to evaluate USP8-mutations in a cohort of MICs–MACs from a unique center and to perform a systematic review and meta-analysis.
Methods
DNA-tumor-tissues from 47 corticotropinomas (16 MICs and 31 MACs) were sequenced. Clinical-biochemical data, radiological imaging data and remission/recurrence rates were evaluated. In addition, we performed a meta-analysis of nine published series (n = 630).
Results
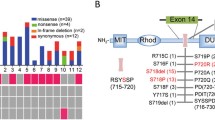
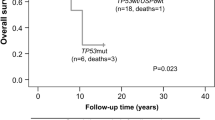
We identified four different USP8-mutations previously described, in 11 out of 47 (23.4%) corticotropinomas; 8 out of 11 were MACs. The urinary cortisol levels of our patients with corticotrophin USP8-mutated-alleles were lower than those of patients with wild-type (WT) alleles (p ≤ 0.017). The frequency of USP8-mutated-alleles among the series was approximately 30% with a higher prevalence in female-patients (p < 0.1 × 10−4). Among the 5 series, the remission rates were higher in patients with USP8-mutated-alleles than in those with the USP8-WT-alleles (p < 0.1 × 10−4).
Conclusion
Our data, as well as the retrospective review of CD series associated with USP8-mutated alleles, show heterogeneous findings among the series. Several drawbacks included the lack of a systematic protocol to evaluate these patients before surgery and follow-up. Further prospective studies using a systematic protocol will provide more consistent information about the influence of the corticotropinomas with USP8-mutated alleles on the phenotype, responses to treatment and outcome of patients with CD.

Similar content being viewed by others
References
Lindholm J, Juul S, Jørgensen JO, Astrup J, Bjerre P, Feldt-Rasmussen U, Hagen C et al (2001) Incidence and late prognosis of cushing’s syndrome: a population-based study. J Clin Endocrinol Metab 86:117–123
Javanmard P, Duan D, Geer EB (2018) Mortality in patients with endogenous cushing’s syndrome. Endocrinol Metab Clin North Am 47:313–333
Dekkers OM, Biermasz NR, Pereira AM, Roelfsema F, van Aken MO, Voormolen JH, Romijn JA (2007) Mortality in patients treated for Cushing’s disease is increased, compared with patients treated for nonfunctioning pituitary macroadenoma. J Clin Endocrinol Metab 92:976–981
Machado MC, Alcantara AE, Pereira AC, Cescato VA, Castro Musolino NR, de Mendonça BB, Bronstein MD et al (2016) Negative correlation between tumour size and cortisol/ACTH ratios in patients with cushing’s disease harbouring microadenomas or macroadenomas. J Endocrinol Invest 39:1401–1409
Nieman LK, Biller BM, Findling JW, Murad MH, Newell-Price J, Savage MO, Tabarin A et al (2015) Treatment of cushing’s syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 100:2807–2831
Bertagna X, Guignat L (2013) Approach to the cushing’s disease patient with persistent/recurrent hypercortisolism after pituitary surgery. J Clin Endocrinol Metab 98:1307–1318
Feelders RA, Hofland LJ (2013) Medical treatment of cushing’s disease. J Clin Endocrinol Metab 98:425–438
Sbiera S, Deutschbein T, Weigand I, Reincke M, Fassnacht M, Allolio B (2015) The new molecular landscape of cushing’s disease. Trends Endocrinol Metab 26:573–583
Reincke M, Sbiera S, Hayakawa A, Theodoropoulou M, Osswald A, Beuschlein F, Meitinger T et al (2015) Mutations in the deubiquitinase gene USP8 cause cushing’s disease. Nat Genet 47:31–38
de Araújo LJ, Lerario AM, de Castro M, Martins CS, Bronstein MD, Machado MC, Trarbach EB et al (2017) Transcriptome analysis showed a differential signature between invasive and non-invasive corticotrophinomas. Front Endocrinol 8:55
Perez-Rivas LG, Theodoropoulou M, Ferraù F, Nusser C, Kawaguchi K, Stratakis CA, Faucz FR et al (2015) The Gene of the ubiquitin-specific protease 8 is frequently mutated in adenomas causing cushing’s disease. J Clin Endocrinol Metab 100:E997–1004
Ma ZY, Song ZJ, Chen JH, Wang YF, Li SQ, Zhou LF, Mao Y et al (2015) Recurrent gain-of-function USP8 mutations in cushing’s disease. Cell Res 25:306–317
Hayashi K, Inoshita N, Kawaguchi K, Ibrahim Ardisasmita A, Suzuki H, Fukuhara N, Okada M et al (2016) The USP8 mutational status may predict drug susceptibility in corticotroph adenomas of cushing’s disease. Eur J Endocrinol 174:213–226
Faucz FR, Tirosh A, Tatsi C, Berthon A, Hernández-Ramírez LC, Settas N, Angelousi A et al (2017) Somatic USP8 gene mutations are a common cause of pediatric cushing disease. J Clin Endocrinol Metab 102:2836–2843
Losa M, Mortini P, Pagnano A, Detomas M, Cassarino MF, Pecori Giraldi F (2019) Clinical characteristics and surgical outcome in USP8-mutated human adrenocorticotropic hormone-secreting pituitary adenomas. Endocrine 63:240–246
Albani A, Pérez-Rivas LG, Dimopoulou C, Zopp S, Colón-Bolea P, Roeber S, Honegger J et al (2018) The USP8 mutational status may predict long-term remission in patients with cushing’s disease. Clin Endocrinol 89(4):454–458
Weigand I, Knobloch L, Flitsch J, Saeger W, Monoranu CM, Höfner K, Herterich S et al (2019) Impact of USP8 gene mutations on protein deregulation in cushing’s disease. J Clin Endocrinol Metab 104(7):2535–2546
Ballmann C, Thiel A, Korah HE, Reis AC, Saeger W, Stepanow S, Köhrer K et al (2018) Mutations in pituitary cushing adenomas-targeted analysis by next-generation sequencing. J Endocr Soc 2:266–278
Machado MC, Fragoso MCBV, Moreira AC, Boguszewski CL, Vieira Neto L, Naves LA, Vilar L et al (2018) A review of cushing’s disease treatment by the Department of Neuroendocrinology of the Brazilian Society of Endocrinology and Metabolism. Arch Endocrinol Metab 62:87–105
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Chaidarun SS, Swearingen B, Alexander JM (1998) Differential expression of estrogen receptor-beta (ER beta) in human pituitary tumors: functional interactions with ER alpha and a tumor-specific splice variant. J Clin Endocrinol Metab 83:3308–3315
Oomizu S, Honda J, Takeuchi S, Kakeya T, Masui T, Takahashi S (2000) Transforming growth factor-alpha stimulates proliferation of mammotrophs and corticotrophs in the mouse pituitary. J Endocrinol 165:493–501
Knosp E, Steiner E, Kitz K, Matula C (1993) Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery 33(4):610–617
Katznelson L, Bogan JS, Trob JR, Schoenfeld DA, Hedley-Whyte ET, Hsu DW, Zervas NT et al (1998) Biochemical assessment of cushing’s disease in patients with corticotroph macroadenomas. J Clin Endocrinol Metab 83:1619–1623
Woo YS, Isidori AM, Wat WZ, Kaltsas GA, Afshar F, Sabin I, Jenkins PJ et al (2005) Clinical and biochemical characteristics of adrenocorticotropin-secreting macroadenomas. J Clin Endocrinol Metab 90:4963–4969
Selvais P, Donckier J, Buysschaert M, Maiter D (1998) Cushing’s disease: a comparison of pituitary corticotroph microadenomas and macroadenomas. Eur J Endocrinol 138:153–159
Losa M, Barzaghi RL, Mortini P, Franzin A, Mangili F, Terreni MR, Giovanelli M (2000) Determination of the proliferation and apoptotic index in adrenocorticotropin-secreting pituitary tumors: comparison between micro- and macroadenomas. Am J Pathol 156:245–251
Chen J, Jian X, Deng S, Ma Z, Shou X, Shen Y, Zhang Q et al (2018) Identification of recurrent USP48 and BRAF mutations in Cushing’s disease. Nat Commun 9:3171
Acknowledgements
We thank Prof. Margaret de Castro for providing us with the USP8-mutated control DNA, and we appreciate all the staff of Laboratorio de Investigacao Medica LIM 42; LIM 25 and Histocell Laboratório de Anatomia Patológica. We also thank Mariana Funari and Vinicius Calsavara for the genetic analysis and meta-analysis, respectively.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) – Finance Code 001 to IQW.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that have no conflicts of interest.
Ethical approval
This study was approved by the Ethical Committee of the Hospital das Clinicas of the University of Sao Paulo, Brazil (#56235216.0.0000.0068) and the procedures were performed in accordance with the 1964 Helsinki Declaration and its later amendments. The signing of informed consent was required.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wanichi, I.Q., de Paula Mariani, B.M., Frassetto, F.P. et al. Cushing’s disease due to somatic USP8 mutations: a systematic review and meta-analysis. Pituitary 22, 435–442 (2019). https://doi.org/10.1007/s11102-019-00973-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-019-00973-9