Abstract
Purpose
There are limited treatment options for progressive atypical pituitary adenomas and carcinomas. Peptide receptor radionuclide therapy that targets somatostatin receptors has recently been proposed as a potential treatment option. The theoretical rationale for efficacy is elegant but evaluation of outcomes in the first patients treated for this indication is required to assess whether further study is warranted.
Methods
We performed a case review of the three pituitary patients we have treated with 177Lutetium DOTATATE in our institution (two atypical adenomas, one carcinoma) and dosimetric analysis of the radiation uptake in one patient.
Results
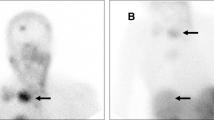
Treatment was well tolerated. One patient with slowly progressive pituitary carcinoma has stable disease 40 months after completing the planned 4 cycles of treatment. Two patients with rapidly progressive atypical adenomas terminated treatment early due to continued disease progression. Dosimetric evaluation revealed inhomogenous uptake across the tumour (1.3–11.9 Gy with one cycle).
Conclusion
We have found mixed results in our first 3 patients with stable disease achieved only in the patient with the more slowly progressive tumour. As only a limited number of centres offer Peptide receptor radionuclide therapy, a formal study with prospective data collection may be feasible and if carried out should include dosimetric evaluation of absorbed dose.




Similar content being viewed by others
References
Saeger W, Ludecke DK, Buchfelder M et al (2007) Pathohistological classification of pituitary tumors: 10 years of experience with the German Pituitary Tumor Registry. Eur J Endocrinol 156:203–216
Zada G, Woodmansee WW, Ramkissoon S et al (2011) Atypical pituitary adenomas: incidence, clinical characteristics, and implications. J Neurosurg 114:336–344
Kaltsas GA, Nomikos P, Kontogeorgos G et al (2005) Clinical review: diagnosis and management of pituitary carcinomas. J Clin Endocrinol Metab 90:3089–3099
Lopes MB, Scheithauer BW, Schiff D (2005) Pituitary carcinoma: diagnosis and treatment. Endocrine 28:115–121
Ortiz LD, Syro LV, Scheithauer BW et al (2012) Temozolomide in aggressive pituitary adenomas and carcinomas. Clinics (Sao Paulo) 67(Suppl 1):119–123
Kaltsas GA, Mukherjee JJ, Plowman PN et al (1998) The role of cytotoxic chemotherapy in the management of aggressive and malignant pituitary tumors. J Clin Endocrinol Metab 83:4233–4238
Colao A, Grasso LF, Pivonello R et al (2011) Therapy of aggressive pituitary tumors. Expert Opin Pharmacother 12:1561–1570
Laws ER Jr, Morris AM, Maartens N (2003) Gliadel for pituitary adenomas and craniopharyngiomas. Neurosurgery 53:255–269 discussion 259–260
Ortiz LD, Syro LV, Scheithauer BW et al (2012) Anti-VEGF therapy in pituitary carcinoma. Pituitary 15:445–449
Ramirez C, Cheng S, Vargas G et al (2012) Expression of Ki-67, PTTG1, FGFR4, and SSTR 2, 3, and 5 in nonfunctioning pituitary adenomas: a high throughput TMA, immunohistochemical study. J Clin Endocrinol Metab 97:1745–1751
Acosta-Gomez MJ, Muros MA, Llamas-Elvira JM et al (2005) The role of somatostatin receptor scintigraphy in patients with pituitary adenoma or post-surgical recurrent tumours. Br J Radiol 78:110–115
Jaquet P, Ouafik L, Saveanu A et al (1999) Quantitative and functional expression of somatostatin receptor subtypes in human prolactinomas. J Clin Endocrinol Metab 84:3268–3276
Taboada GF, Luque RM, Bastos W et al (2007) Quantitative analysis of somatostatin receptor subtype (SSTR1-5) gene expression levels in somatotropinomas and non-functioning pituitary adenomas. Eur J Endocrinol 156:65–74
Saveanu A, Gunz G, Guillen S et al (2006) Somatostatin and dopamine-somatostatin multiple ligands directed towards somatostatin and dopamine receptors in pituitary adenomas. Neuroendocrinology 83:258–263
Hofland LJ, Lamberts SW, Feelders RA (2010) Role of somatostatin receptors in normal and tumoral pituitary corticotropic cells. Neuroendocrinology 92(Suppl 1):11–16
Hofland LJ, Feelders RA, de Herder WW et al (2010) Pituitary tumours: the sst/D2 receptors as molecular targets. Mol Cell Endocrinol 326:89–98
Fusco A, Giampietro A, Bianchi A et al (2012) Treatment with octreotide LAR in clinically non-functioning pituitary adenoma: results from a case-control study. Pituitary 15:571–578
Rahmim A, Zaidi H (2008) PET versus SPECT: strengths, limitations and challenges. Nucl Med Commun 29:193–207
Kuyumcu S, Ozkan ZG, Sanli Y et al (2013) Physiological and tumoral uptake of (68)Ga-DOTATATE: standardized uptake values and challenges in interpretation. Ann Nucl Med 27:538–545
Kunikowska J, Krolicki L, Pawlak D et al (2012) Semiquantitative analysis and characterization of physiological biodistribution of (68)Ga-DOTA-TATE PET/CT. Clin Nucl Med 37:1052–1057
Boy C, Heusner TA, Poeppel TD et al (2011) 68 Ga-DOTATOC PET/CT and somatostatin receptor (sst1–sst5) expression in normal human tissue: correlation of sst2 mRNA and SUVmax. Eur J Nucl Med Mol Imaging 38:1224–1236
Shastry M, Kayani I, Wild D et al (2010) Distribution pattern of 68 Ga-DOTATATE in disease-free patients. Nucl Med Commun 31:1025–1032
Veit JA, Boehm B, Luster M et al (2013) Detection of paranasal ectopic adrenocorticotropic hormone-secreting pituitary adenoma by Ga-68-DOTANOC positron-emission tomography-computed tomography. Laryngoscope 123:1132–1135
Kwekkeboom DJ, de Herder WW, Kam BL et al (2008) Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0, Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol 26:2124–2130
van Essen M, Krenning EP, Kooij PP et al (2006) Effects of therapy with [177Lu-DOTA0, Tyr3]octreotate in patients with paraganglioma, meningioma, small cell lung carcinoma, and melanoma. J Nucl Med 47:1599–1606
Bartolomei M, Bodei L, De Cicco C et al (2009) Peptide receptor radionuclide therapy with (90)Y-DOTATOC in recurrent meningioma. Eur J Nucl Med Mol Imaging 36:1407–1416
Baldari S, Ferrau F, Alafaci C et al (2012) First demonstration of the effectiveness of peptide receptor radionuclide therapy (PRRT) with 111In-DTPA-octreotide in a giant PRL-secreting pituitary adenoma resistant to conventional treatment. Pituitary 15(Suppl 1):S57–S60
Komor J, Reubi JC, Christ ER (2013) Peptide receptor radionuclide therapy in a patient with disabling non-functioning pituitary adenoma. Pituitary. doi:10.1007/s11102-013-0494-0
Siegel JA, Thomas SR, Stubbs JB et al (1999) MIRD pamphlet no. 16: techniques for quantitative radiopharmaceutical biodistribution data acquisition and analysis for use in human radiation dose estimates. J Nucl Med 40(2):37S–61S
Pernicone PJ, Scheithauer BW, Sebo TJ et al (1997) Pituitary carcinoma: a clinicopathologic study of 15 cases. Cancer 79:804–812
Salehi F, Agur A, Scheithauer BW et al (2009) Ki-67 in pituitary neoplasms: a review–part I. Neurosurgery 65:429–437 discussion 437
Janssen MH, Ollers MC, van Stiphout RG et al (2012) PET-based treatment response evaluation in rectal cancer: prediction and validation. Int J Radiat Oncol Biol Phys 82:871–876
van Elmpt W, Ollers M, Dingemans AM et al (2012) Response assessment using 18F-FDG PET early in the course of radiotherapy correlates with survival in advanced-stage non-small cell lung cancer. J Nucl Med 53:1514–1520
Haug AR, Auernhammer CJ, Wangler B et al (2010) 68 Ga-DOTATATE PET/CT for the early prediction of response to somatostatin receptor-mediated radionuclide therapy in patients with well-differentiated neuroendocrine tumors. J Nucl Med 51:1349–1356
Kwekkeboom DJ, Teunissen JJ, Bakker WH et al (2005) Radiolabeled somatostatin analog [177Lu-DOTA0, Tyr3]octreotate in patients with endocrine gastroenteropancreatic tumors. J Clin Oncol 23:2754–2762
Hanscheid H, Sweeney RA, Flentje M et al (2012) PET SUV correlates with radionuclide uptake in peptide receptor therapy in meningioma. Eur J Nucl Med Mol Imaging 39:1284–1288
Cremonesi M, Botta F, Di Dia A et al (2010) Dosimetry for treatment with radiolabelled somatostatin analogues. A review. Q J Nucl Med Mol Imaging 54:37–51
Hindorf C, Chittenden S, Causer L et al (2007) Dosimetry for (90)Y-DOTATOC therapies in patients with neuroendocrine tumors. Cancer Biother Radiopharm 22:130–135
Kaltsas GA, Papadogias D, Makras P et al (2005) Treatment of advanced neuroendocrine tumours with radiolabelled somatostatin analogues. Endocr Relat Cancer 12:683–699
Villard L, Romer A, Marincek N et al (2012) Cohort study of somatostatin-based radiopeptide therapy with [(90)Y-DOTA]-TOC versus [(90)Y-DOTA]-TOC plus [(177)Lu-DOTA]-TOC in neuroendocrine cancers. J Clin Oncol 30:1100–1106
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maclean, J., Aldridge, M., Bomanji, J. et al. Peptide receptor radionuclide therapy for aggressive atypical pituitary adenoma/carcinoma: variable clinical response in preliminary evaluation. Pituitary 17, 530–538 (2014). https://doi.org/10.1007/s11102-013-0540-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-013-0540-y